Purity, Pipes, and Plumbing: The Loss Of Mineral Order In Your Water
R.O. and distillation of water made "pipe-friendly" by regulatory standards then strips away the mineral architecture that life evolved with. What remains looks purer but is harmful to your health.
Aurmina End-of-Year Sale
As we close out Aurmina’s first year, really, its first few months, we wanted to mark the moment with something simple: gratitude.
Lisa, Scott, and I built Aurmina because we believed there was a missing piece in modern water treatment, and we’ve been genuinely moved by how many people immediately understood what we were trying to do. Watching this small company help real people has been deeply satisfying, and we wanted to say thank you.
Through the end of the year, we’re offering 25% off single bottles, with no limit on quantity. For those who already know they’ll be using Aurmina long-term, the 6-pack remains the best value at 34% off, so don’t outsmart yourself by buying six singles.
Discount Code: HOLIDAY
If you’ve been following my water series, you already know why this exists. Aurmina isn’t about stripping water down to nothing. It’s about restoring order, clarity, and purity after modern treatment has done its job.
And in the spirit of transparency (and a little humor), the first draft of the post below was written before Aurmina even existed. If that makes this post a marketing scheme, it’s the most accidental one I’ve ever been part of.
Either way, this is our thank-you. The sale runs through the end of the year.
Modern water is engineered for infrastructure, not for life. Whether softened, hardened, R.O’ed, or distilled, the processes that make it behave in pipes steadily remove the mineral order biology depends on.
Today I want to walk through why and how stripped-down water (reverse osmosis and distilled) can quietly drive a wide range of detrimental health effects.
R.O, Distilled, and High-Grade Filtered Water: Solving One Problem While Creating Another
Many “health enthusiasts” mistakenly believe that high-grade gravity filtration, reverse osmosis, or distilled water is an improvement to their health by removing any of the dozens of contaminants we have covered in previous chapters. The reality is that while these methods do largely remove contaminants, they also render water biologically inert by stripping essential trace and major minerals.
Life as we know it is inseparable from minerals. Water provides the medium, but mineral ions provide the function. Without minerals, water can no longer participate meaningfully in biology.
Alarmingly, mineral-free water is actually detrimental to health. This should not be surprising because no natural source of drinking water on Earth, now or historically, has ever been devoid of dissolved minerals or organic material.
Remember, we live on a rock. Thus all groundwater, surface water, springs, rivers, and even glacial melt all carry ionic mineral content. Human physiology evolved on mineralized water, not on a chemically stripped solvent.
Human biology reflects this dependency. When mineral-free water enters the gut, it cannot be absorbed as is. Electrolytes must first be added, drawn from the body’s own reserves. Numerous controlled studies show that consumption of demineralized water increases urine output and accelerates renal loss of sodium, potassium, calcium, and magnesium. Serum potassium levels fall, hormonal regulation of fluid balance shifts, and water redistributes out of cells into plasma. The body must surrender its own minerals simply to make the water usable.
Why Demineralized Water Poorly Hydrates Cells
Water does not hydrate the body by volume alone. It hydrates by electrochemistry. For water to enter cells, remain there, and participate in metabolism, it must carry dissolved ions.
Mineralized water arrives with electrolytes already in solution, allowing it to follow osmotic and electrical gradients into tissues. Demineralized water does not. When reverse-osmosis or distilled water enters the gut, it must first acquire ions to become absorbable, and it can only obtain them from the body itself.
The result is increased diuresis, accelerated loss of sodium, potassium, calcium, and magnesium, and redistribution of water out of cells into plasma. In practical terms, mineral-free water passes through the body efficiently, but it does not hydrate efficiently.
This is why zero-TDS water consistently behaves as hypotonic and physiologically unstable. Lacking buffering ions, especially bicarbonate, it increases renal electrolyte loss, destabilizes plasma osmolality, and forces continuous hormonal correction rather than allowing the system to relax into hydration.
No natural drinking water source on Earth is mineral-free, because biology did not evolve to hydrate on chemically stripped solvent. Hydration is retention, not intake, and retention depends on mineral context. Water without ions may be analytically pure, but inside a living system it behaves as transient fluid rather than as a biological medium.
*A major (but not the only) source for the following comes from this comprehensive review by František Kožíšek, M.D., Ph.D., published by the Czech National Institute of Public Health in 2004, which also included previous reviews done by the WHO.
Bicarbonate, TDS, and the Architecture of Physiologic Water
One of the most under-appreciated consequences of “demineralized” water, meaning reverse osmosis (R.O.) or distilled water, is that both processes remove bicarbonate almost completely and dramatically lower total dissolved solids (TDS) towards zero.
The bulk of TDS in our drinking water is from geologically derived minerals (good) and mineral compounds generated by pipes, treatment chemicals, and pollution (not so good). The remainder of the TDS are from metals and “organic compounds,” meaning all the substances people fear, such as pesticides, disruptors, disinfection byproducts, and treatment-derived chemicals. When TDS decreases towards zero, it shows that these processes remove all of the “bad stuff” but also all of the “good stuff.”
Looking at a TDS number alone does not tell you much, because two waters with the same TDS can have dramatically different content dissolved within. Although the statement that adding Aurmina will not significantly change the TDS number is true, what it does do is change the TDS profile from a “bad” one to a “good” one, meaning that, via flocculation and precipitation, it will remove the components you don’t want, such as organics, treatment chemicals, and disinfectants, and will leave behind the ones you do want, namely geologically derived minerals, in a ratio and composition that increases electrical conductivity and allows for structuring.
Because bicarbonate exists only in dissolved equilibrium with carbon dioxide and mineral cations, any technology that strips ions indiscriminately, whether using membranes, distillation, or deionization, will leave your water with near-zero buffering capacity.
Typical R.O. or distilled water contains less than 5–10 mg/L bicarbonate, often effectively zero. This matters because bicarbonate is not a cosmetic mineral. It is the dominant buffering system in human physiology, tightly linked to acid–base balance, renal handling of electrolytes, calcium solubility, and vascular tone.
WHO-reviewed studies show that water with low bicarbonate content increases diuresis and electrolyte loss, while waters containing moderate to high bicarbonate, approximately 80–250 mg/L, were associated with lower morbidity and better metabolic stability.
In other words, R.O. and distilled water are chemical and mineral free, but physiologically incomplete. They remove not only contaminants, but also the buffering architecture that allows water to interact gently and coherently with biology.
So, How Much Bicarbonate and TDS Should Be In My Water?
A large Russian ecological study compared regions given “low-mineralized” and “high-mineralized” water, and found that the lowest morbidity was associated with water having calcium levels of 30–90 mg/L, magnesium levels of 17–35 mg/L, a bicarbonate level between 200–250 mg/L and a TDS of about 400 mg/L. The author concluded that such water could be considered as “physiologically optimum.”
How Aurmina Balances Without Bicarbonate
Aurmina-treated water does not function by adding bicarbonate directly, nor does it attempt to mimic mineral water through forceful ion supplementation. It operates upstream, at the level of mineral order, redox balance, and charge organization, overlapping functionally with the stabilizing roles bicarbonate plays in natural waters.
Bicarbonate stabilizes water by buffering pH, coordinating divalent cations, moderating redox reactivity, and preventing aggressive ionic behavior. Aurmina on the other hand, achieves similar stabilizing effects through a different mechanism: removing disordered mineral load, collapsing colloids, coordinating multivalent ions, and restoring electrical and redox coherence in the remaining water.
In practical terms, even when measured bicarbonate does not rise dramatically, the water behaves as though buffering capacity has returned. pH stabilizes. Oxidation–reduction potential normalizes. Electrical conductivity improves. Exclusion-zone formation becomes detectable. Carbonate–CO₂ equilibria re-establish themselves under calmer, more coherent conditions.
The result is water that is neither stripped and aggressive like R.O. or distilled water. It is redox-stable, mineral-coordinated, and structurally permissive.
“It’s All Good, I Have A Remineralization Filter “
Yeah right. If you think your remineralization filter is making your water healthy, or even physiologic, you would be “dead” wrong (just like your water, sorry). Standard remineralization filters used with R.O systems produce demonstrably suboptimal TDS and bicarbonate levels.
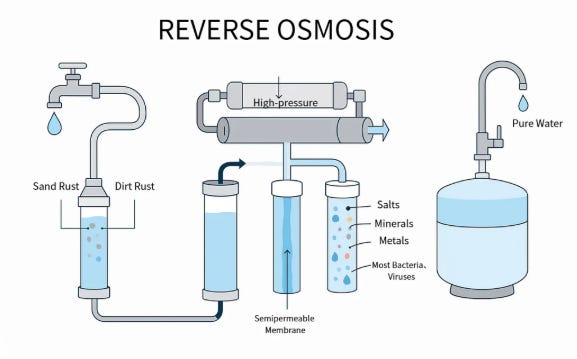
Just like in yesterday’s post where we walked along your waters journey from aquifer to municipal treatment plant then to your house, now let’s walk through the journey that your water takes when it meets your R.O system.
R.O Filtration Step
Upon passing through the R.O filters, 90–99% of dissolved solids are removed, including calcium, magnesium, bicarbonate, sulfate, sodium, and unfortunately, trace elements as well.
What remains is mostly residual CO₂, trace sodium or silica, and very small amounts of whatever the membrane leaks.
Typical TDS readings at this point range from 5–30 mg/L (ideal is around 400mg/L), typical bicarbonate level is 0–10mg/L (ideal is around 224 mg/L). Functionally, at this point, your R.O. water is low-buffer, low-ionic, and electrically weak, even when it’s analytically “clean.”
Remineralization Filter Step
Your remineralization filter then tries to “build it back up again” by using a paltry, narrow set of salts like Calcite (CaCO₃), Corosex (MgO), limestone blends, and occasionally dolomite (CaMg(CO₃)₂). This raises your bicarbonate on average to between 30–80 mg/L (ideal around 225), and your TDS to 50–100 ppm TDS (ideal around 400).
Thus, with or without remineralization filters, your R.O water will have a low TDS and low bicarb. There are two problems with that;
salts are leached from the body under the influence of drinking water with a low TDS
water with a TDS of 25–50 mg/L is described as tasteless in volunteer studies
water-salt balance is altered when TDS is between 50 and 75 mg/L, thus the WHO recommends that the minimum TDS in drinking water should be 100 mg/L.
Thus, even with remineralization filters, your water falls way short. Also know that two waters can have the same TDS and behave nothing alike. For instance, 50 mg/L from calcite chips is not equivalent to 50 mg/L from natural spring minerals, or even 100 mg/L from CaCO₃ alone has nothing to do with 100 mg/L distributed across multivalent, coordinated ions like in Aurmina.
Ultimately, TDS tells you quantity, not organization. Industry remineralization is focused on raising TDS just enough to avoid corrosion, but it does not restore redox balance, trace mineral spectra, or structured, biologically coherent water. It solves a plumbing problem, not a physiological one.
How Does U.S Municipal Water Fare?
Typical U.S. municipal water shows enormous variability, but most systems fall roughly into these ranges. Let’s start with bicarbonate:
Soft surface water systems: ~20–60 mg/L HCO₃⁻
Moderately buffered systems: ~60–120 mg/L
Hard groundwater systems (Midwest, Southwest): 150–300+ mg/L
Thus, many U.S. systems, especially those using reverse osmosis, ion exchange softening, or aggressive corrosion control, operate below the bicarbonate levels associated with lowest morbidity.
Now lets look into TDS, where U.S systems fare a little better:
The nationwide averages range from 150–400 mg/L, with a median U.S. municipal TDS of around 250 mg/L. By source type, surface water systems range between 50–200 mg/L, while groundwater systems range higher, from 200–600 mg/L, largely due to prolonged water–rock interaction.
Health Impacts of Drinking De-Mineralized Water
If you’re an R.O or distilled water drinker and you’re still with me, you may wish you weren’t as sometimes ignorance is bliss. Unfortunately (or fortunately), there is a substantial body of scientific evidence that documents adverse health effects associated with consumption of demineralized water. These effects are not theoretical and they do not require decades to manifest.
Clinical studies report fatigue, weakness, muscle cramps, headaches, and disturbances in cardiac rhythm appearing within weeks to months of consuming reverse osmosis or distilled water, particularly in relation to magnesium and calcium depletion. These findings were significant enough that multiple European public-health authorities issued formal warnings against routine consumption of distilled water.
Even with “lightly mineralized” water, major health disturbances can be measured. The largest study in the literature comes from Russia, where they compared two regions in Russia with the same eating habits, air quality, social conditions, and time of residence.
One area received low-mineral water, (all units in mg/L): TDS-134, Calcium-18.7, Mag-4.9, Bicarb-86, while the other area received high mineral water, TDS-385, Ca-29.5, Mag-8.3, Bicarb-243.
Findings:
Adults drinking low-mineral water had higher rates of hypertension, ischemic heart disease, gastric and duodenal ulcers, chronic gastritis, cholecystitis, and nephritis
Children in the low-mineral area showed slower physical development and increased growth abnormalities
Pregnant women had higher rates of edema and anemia
Newborns in the low-mineral area had higher overall morbidity
From the comprehensive review by František Kožíšek, M.D., Ph.D;
“Consuming R.O. water for even a few months can lead to side effects such as fatigue, weakness, muscle cramps, and potentially impaired heart rhythm due to low levels of magnesium and calcium.”
Epidemiological studies link long-term consumption of low-mineral water to cardiovascular disorders, hypertension, osteoporosis, and complications during pregnancy and infancy. Mineral loss is further exacerbated during food preparation, as R.O. water can leach minerals from foods cooked in it, further reducing dietary intake.
Communities consuming low-calcium and low-magnesium water consistently demonstrate higher rates of cardiovascular disease, stroke, hypertension, and sudden cardiac death. Magnesium deficiency, in particular, destabilizes cardiac electrical activity in ways that mineral-free water quietly amplifies.
There is another rarely discussed consequence. Mineral-free water is chemically aggressive. Lacking buffering ions, it readily dissolves metals from pipes, fittings, storage tanks, and fixtures. Documented cases of lead exposure, including infant poisoning, have been traced to low-mineral water leaching metals from household plumbing. In attempting to remove contaminants, purified water can create new exposure pathways the moment it enters real-world infrastructure.
The Hard Water vs. Soft Water Problem
Hard water is water that is rich in calcium and magnesium, typically acquired through prolonged contact with limestone, dolomite, or other mineral-rich geology. The modern (not historical) concerns over hard water is that it can form scale in pipes, boilers, and kettles, reduces soap lather, leaves residue on fixtures, and causes appliance inefficiency over time. Cavemen did not have those concerns.
But, from a biological perspective, hard water supplies dietary calcium and magnesium, which often correlates with lower cardiovascular mortality in population studies. It also provides buffering capacity via bicarbonate–carbonate systems and thus is generally less corrosive to pipes, which is important for lead and copper exposure.
Know that since free protons drive many calcification processes, Aurmina, by its ability to moderate proton activity, makes calcium stay in a more stable, usable state instead of converting into hard, insoluble deposits.
Although you can’t use Aurmina as an addition to the plumbing infrastructure of your home, when you use Aurmina treated water for your plants and soil, the calcium stays more accessible to plant roots instead of binding tightly and becoming unavailable. For people prone to kidney stones, calcium that is less inclined to crystallize is generally better tolerated than calcium that readily calcifies.
Soft water on the other hand, is water that is low in calcium and magnesium. In practical terms, this results in better soap performance and no scale formation, but such water is often more aggressive and corrosive chemically.
From a biological perspective, naturally soft water has low mineral contribution, often has low buffering capacity, and can enhance metal leaching from pipes, increasing exposure to lead, copper, and iron. Soft does not mean clean or healthy; it simply means low hardness.
Epidemiologic studies from Europe and Russia repeatedly found higher rates of cardiovascular disease and sudden cardiac death in regions with very soft water, likely due to magnesium deficiency.
Threading The Needle
So we have a “tough needle to thread” here, meaning if your water is hard, although it is better for your health in terms of being a source of minerals and protecting you from pipe corrosion, it increases risks of scaling in your plumbing.
If your water is soft, it is not good for your health because it lowers mineral intake and may increase exposure to metals in your pipes, however, you don’t have to worry about scaling.
So what do people do? Soft water people “harden” their water, hard water people “soften.” Let’s walk through what that looks like. And it ain’t good.
Artificially Softening Water (Ion Exchange)
This is where most confusion arises because ion-exchange softeners actually make things worse. They remove calcium and magnesium and replace them with Sodium (Na⁺) or Potassium (K⁺), which results in water with zero hardness, higher sodium, same TDS or higher, no buffering improvement, and no biological magnesium.
From a biological perspective, it is worse for people with hypertension, kidney disease, and heart failure. Further, it remains chemically aggressive, and certainly not mineral-restorative, just mineral-substituted.
Artificially Hardening Water
Converse to hard water, soft water is typically low in calcium and magnesium and often slightly acidic. Municipalities and homeowners “harden” water primarily to protect infrastructure, improve taste, stabilize pH.
Various methods are employed to harden water:
Calcium And Bicarbonate
Most common is adding calcium carbonate (CaCO₃), calcium hydroxide (lime), and sodium bicarbonate or calcium bicarbonate (for alkalinity). The pros are that it reduces pipe corrosion, raises pH and alkalinity, and improves taste. The cons are that the minerals are often added in simple ionic forms and can increase carbonate without restoring mineral balance. Thus it produces water that is harder but not necessarily biologically coherent. This often creates disordered hardness.
Crushed Limestone Or Calcite
Another method used by homes and municipalities is to pass water through crushed limestone or calcite media which adds calcium naturally and raises alkalinity gently. The problem is that this adds mostly calcium, not magnesium or trace minerals. You can overshoot hardness while not restoring trace mineral diversity. This has a limited effect on redox balance. Better than nothing, but incomplete.
Dolomite Media (Calcium + Magnesium)
Some systems use dolomite (calcium and magnesium) instead of pure calcite which produces a better mineral ratio than calcite alone, but you are still limited to bulk minerals with absent trace elements and the mineral form depends heavily on water chemistry. This can approach partial order, but it’s still blunt.
DIY Mineral Addition
Some people add baking soda (sodium bicarbonate), Epsom salt (magnesium sulfate), calcium chloride or commercial mineral drops. The pros are that it is cheap, adjustable, and can raise alkalinity quickly but the cons are that it is very easy to overdose, it creates ionic chaos, and often worsens redox instability. Also it can aggravate GI, renal, or cardiovascular issues. This is the fastest way to turn soft water into disordered hard water.
“Ordered vs. Disordered” Water Rather Than “Hard vs. Soft” Water
Biologically and physically, because hardness refers to calcium and magnesium content it is a signal that your water has spent time in contact with mineral lattices that confer charge balance, buffering capacity, and redox stability. Ultimately, hard water should be considered an entry point and not an endpoint.
Yes, without it, you cannot have organized water, but at the same time, most modern “hard water” will not allow for optimal organization.
The biologically meaningful distinction in water is therefore not “hard versus soft,” but “ordered versus disordered.” Ordered mineral water retains minerals in forms that support redox balance, charge separation, and structural coherence. Disordered mineral water contains the same elements stripped from their natural context, behaving as reactive stressors rather than biological supports.
Modern water treatment excels at producing chemically compliant water, but often at the cost of mineral order, the very property life depends upon.
Where Aurmina Comes In For All Camps
Aurmina doesn’t care whether your water started out hard or soft, stripped or overloaded, piped through copper or pulled from a well. It steps in after all of that and does the one thing modern water systems never even attempt: restore order.
Hard-water people spend their lives trying to soften water so it behaves in pipes. Soft-water people spend theirs trying to harden it for taste and corrosion control. R.O. and distilled users swing the pendulum to zero, then work to bolt minerals back on. Every one of these strategies is aimed at managing plumbing and pollution. None of them are designed around physiology.
Aurmina bypasses that entire argument by focusing on the water you actually ingest, not the water rushing through municipal infrastructure. Water optimized for pipes is engineered to remain chemically tame, visually clear, and legally compliant.
Water optimized for biology must be mineral-ordered, redox-balanced, and structurally coherent. Aurmina removes mineral chaos without stripping life out of the water, allowing structure to emerge rather than forcing chemistry in.
What you end up with is water that looks and acts like what comes out of the best mineral springs on Earth. Now you know why I started this company :).
Aurmina doesn’t ask you to change how your city treats water or how your house is plumbed. It optimizes the final interface between water and biology. Your body is not a pipe. Stop trying to make your drinking water behave like an industrial fluid.
Conclusion
By this point, the scope of the problem is clear. Modern drinking water is burdened, imperfectly regulated, and chemically altered in ways that rarely enter public discussion. We’ve examined contamination, delayed exposure, misplaced confidence in bottled and “purified” water, and the negative health consequences of long-term mineral absence.
In the next chapter, we step away from regulations, reports, and assumptions and into something less abstract. We let the water show what it has been carrying, and what falls out when Aurmina chemistry finally allows it to happen. The long awaited “Sediment Analysis” post, coming to you tomorrow…
If you value the late nights and deep dives into all the “rabbit holes” I then write about (or the Op-Eds and lectures I try to get out to the public), supporting my work is greatly appreciated.
More Stuff (Aurmina and Book Publications)
If you want to learn more about the water purifier we made from Shimanishi’s volcanic-mineral complex, go to Aurmina.com where we are running a 25% off end-of year sale.
Upcoming Book Publications
Yup — not one, but two books are dropping from yours truly (at the same time? What?)
If, instead of (or in addition to) these Substack posted chapters, you prefer the feel of a real book, or the smell of paper, or like to give holiday gifts, pre-order From Volcanoes to Vitality, my grand mineral saga, shipping end of January.
And if you want to read (or gift) another chronicle of suppression, science, and survival, grab The War on Chlorine Dioxide—the sequel you didn’t see coming—shipping early to mid-January. On this one, I say: “Buy it before they ban it.” Hah!




Very important and well written article. I should say, critically important!
I have been cooking with a sourdough starter for about 15 or so years. Sometimes sourdough starter can be a struggle. As I’ve learned more along my sourdough journey, I have taught small casual classes to friends and family and sometimes in the community.
About 5 or 6 years ago, maybe longer, I was using some spring water while staying at a remote cabin in the mountains. My starter was SO happy. Noticeably happier than usual. From that time, I would take drives to the mountains to fill containers of spring water just to use with my sourdough starter and it made such a difference.
I don’t always have the time now to drive out to fill containers with spring water so I found that store bought spring water works just as well. When I teach classes now, I encourage people to use spring water, especially if they’ve tried sourdough and haven’t had good results.
I have thought many times that if my sourdough thrives on spring water, but not on my tap water (well water that has been softened), maybe spring water is what humans should be drinking as well.
My bottle of aurmina arrives any day. I’ll definitely be experimenting with it to feed my sourdough starter to see how it behaves. And I’m looking forward to any healthy benefits that it may bring to me and my family. Thank you!