Our Reply To the American Board Of Internal Medicine
76 pages, 22,000 words, 176 references, 11 exhibits of data to support our many public statements. All for naught.
JOINT STATEMENT OF PIERRE KORY, M.D., MPA AND PAUL E. MARIK, M.D., FCCP, FCCM* TO THE AMERICAN BOARD OF INTERNAL MEDICINE
Response to the Board’s May 26, 2022 Notice of Potential Disciplinary Action
January 5, 2022
* Please note that there is also an Addendum Statement regarding an additional issue raised regarding Dr. Marik.
TABLE OF CONTENTS
Section 1: Opening Statement ........................................................................................................ 1
I.......... Use by ABIM of Sanctions Based on its Own Independent Judgment about Disinformation is a Novel and Original Action that Requires Care as to Procedure; Consideration Must be Given of its Impact on the Development of Medicine; Further We Preserve Objections Regarding Standard of Care and Scope of the Inquiry........................................................ 2
II........ The Standard of Review Should be Clearly Articulated as Unsupported By Evidence Rather than Merely Disagreement with Public Health Messaging or Majoritarian Viewpoints............................................................................... 2
III....... Any Adverse Decision Should Detail the Nature of the Evidence Presented Rather than Merely state Disagreement with Public Health or other Medical “Authorities”; The ABIM Should Remain Independent and Allow Diverse Opinion. 4
IV............................................. Avoiding Arbitrary, Capricious, and Inconsistent Determinations. 5
V...................................... A Note about Legal Restrictions on Speech and Other Legal Concerns. 6
Section Concluding Thoughts ............................................................................................. 6
Section 2: The Work of the FLCCC and Respondents’ Credentials and Expertise........................ 7
Section 3: Recommendations Made about the Use of Ivermectin in COVID-19 are Evidence-Based and Appropriate 10
I.......... There is Substantial Evidence That Ivermectin Has Significant Clinical Utility in Treating COVID 10
A........... Peer-reviewed, published evidence supports the use of ivermectin in COVID-19. 10
B. ............................................. Epidemiologic Evidence Provides Further Strong Support. 12
C. ...... Ivermectin as part of a Protocol Has Been Shown to Have Effectiveness as a COVID-19 Prophylactic. 13
D........ FLCCC, Dr. Kory and Dr. Marik have emphasized that ivermectin is most effective as part of a protocol. 14
II........ Contrary Views are Based in Part on Misunderstanding Public Agency Positions about Safety and Efficacy, Unfounded Criticisms, and a Bias against Repurposed Drugs......................................................................................... 15
A........ The FDA is incorrectly perceived as stating that physician prescribing Ivermectin for COVID-19 is improper. 15
1) ....... The FDA does not set standards of care for the off-label use of drugs..... 15
2) ....... “Off-label” use of drugs does not indicate failure to meet standard of care. 15
3)........ The FDA’s “You are not a horse” and other campaigns did not state an FDA position against such prescribing 16
4) ....... The FDA has not studied the use of ivermectin in COVID-19 and reached no conclusion that its use in COVID-19 is not safe or effective......................................................................................... 17
5) ....... The FDA has expressly disavowed any intention to set the standard of care for the use of ivermectin in COVID-19. 17
B................ The Centers for Disease Control and the Facts about the Safety of Ivermectin. 17
C........ The NIH Position Has Shifted Several Times and its Guidelines Do Not Support Discipline. 20
D. ...... The AMA position and other Echo Chambers Provide no Support for Action against Dr. Kory or Dr. Marik. 21
III.......................................... The Ivermectin Critiques Do Not Undercut its Demonstrated Value. 22
A............... Published Metastudies Reaching Contrary Conclusions are not Well-founded. 22
B........................................................ Criticism of Favorable Studies is not Well-Founded. 23
C..................................... Null Studies Purporting to No Results Have Substantial Defects. 23
IV.................................................................... Safety Comparisons with Other Treatment Options. 25
V........ The Validation of Repurposed Drugs Faces Nearly Insurmountable Real World Challenges. 26
Section Concluding Thoughts............................................................................................ 29
Section 4: Hydroxycholoroquine and other Repurposed Drugs................................................... 30
I.......... There is Sufficient Evidence to Recommend Hydroxychloroquine, Particularly When Viewed Absent to Political Tropes Surrounding its Use............................................................................................... 30
Section Concluding Thoughts............................................................................................ 31
Section 5: Dr. Kory’s and Dr. Marik’s Statements about Vaccination are Evidence-Based, Entirely Appropriate, and not Disinformation 32
I. ........ Clinical Evidence for mRNA Vaccine Risks Are Significant and Have Been Underplayed. 33
A........ There is substantial data that “all-cause” mortality has been elevated by the mRNA vaccine. 33
B........ V-Safe Data Shows Significant Safety Concerns that Public Health Authorities have not Communicated to the Public. 34
C.......................................................... VAERS Data Reveals Significant Safety Concerns. 37
D................................ Epidemiologic Data Demonstrates Highly Alarming Safety Signals 39
E......... There are substantial risks associated with receipt of a COVID-19 mRNA vaccination, particularly in younger patients, which have been globally recognized by a number of governments who are at odds with the CDC position. 40
II........ Real World Efficacy Data Raises Serious Concerns about the Benefit of mRNA Vaccines and Demonstrates that Vaccinated Individuals Are Likely at Higher Risk................................................................................................. 47
A. ........................................................ Numerous Studies Demonstrate Negative Efficacy. 47
B........ The Evidence Alleging to Show Significant Reduction in Severity and Mortality from the mRNA Vaccine Is Overstated. 53
C........ The Evidence Also Demonstrates that Vaccination is Not Effective in Preventing Severe Disease 55
D........................... Vaccination Also Does Not Appear to Prevent Long-Haul COVID-19. 58
III....... Natural Immunity Appears to Be Superior than mRNA Vaccination And, in Light of Potential Risks, Supports the Reasonable Position That the Vaccine Should Be Deferred in Patients with a Positive History....................... 59
IV....... The ABIM Considerations Should Take into Account that other Official Governmental Actions Recognize these Issues and Stand in Contrast to Federal Public Health Policy. ........................................................................ 60
V............................................................... Legal Aspects of Vaccine Policy and Physician Speech. 60
Section Concluding Thoughts............................................................................................ 61
Section 6: Public Positions Regarding these Controversies........................................................ 62
I.......... Positions taken by governmental and other established agencies are contrary to the proposed action by the ABIM. 62
A........................ Support from the Office of the Nebraska Office of the Attorney General. 62
B........ States are passing or proposing legislation for “Off Label” Use of ivermectin which demonstrate both public opinion and considered views that such conduct should be allowed without imposition of consequences. 62
Section 7: The Article Retraction Cited in the Notice is Not a Basis for Discipline.................... 64
I.................... The Article of Concern was Subjected to Additional Peer Review and Republished. 64
II........ The Original Retraction Grew Out of An Employment Dispute, and the Usual and Customary Efforts to Correct a Minor Concern Were Rejected............................................................................................................................. 64
III. .......... The Original Journal Asked for and Rejected Additional Studies on Baseless Grounds. 65
IV....... The Article was also Criticized By Comparing Studies with Null Results: These Differences can be Explained by Flaws in Study Design, Particularly Treatment Delay............................................................................................ 67
Section Concluding Thoughts ........................................................................................... 68
Statement Conclusion................................................................................................................... 68
Section 1
Opening Statement
We believe it is incumbent upon physicians to honestly examine the medical evidence. In the case of repurposed uses for drugs, that requires accounting for structural obstacles and conflicts of interest that oppose such uses in favor of new drug development. It requires objective consideration of all evidence, informed by clinical experience, rather than result-oriented metastudies that exclude favorable studies on specious grounds,
In the case of vaccine policy, it requires an awareness that early adoption of a novel vaccine approved under exigent circumstances requires attention to post-approval surveillance, assessment of actual risks and benefits, and independence from public health messaging whose aim is overtly designed to gain program compliance. Independent assessment is all the more necessary when public health authorities admit little reduction in transmission and less benefit than originally projected while, at the same time, numerous concerns about ill effects are revealed. That the CDC and FDA were unwilling to release critical data and only did so under court order increases the importance of such independent examination. In this Statement, we document at length the basis for Dr. Kory’s and Dr. Marik’s statements in the medical evidence, are reasonably held, and do not constitute “disinformation.”
We believe that the proper role of the American Board of Internal Medicine (“ABIM” or “Board”) is to further the professional integrity of medicine by encouraging evidence-based debate rather than enforcing public health messaging. Dr. Kory and Dr. Marik do not stand alone but cite a large number of peer-reviewed published studies, state governments, and other nations in support of their positions. The ABIM’s use of its power to sanction diplomates by framing disagreement as disinformation has raised considerable alarm, and there is an open letter signed by over a thousand physicians and other individuals specifically protesting this proposed action. “An open letter to the American Board of Medical Specialties and the Federation of State Medical Boards: The destruction of Member Boards’ credibility.”[1] (Exhibit A).
This concern is well-founded given that the Board would consider taking action against these eminent physicians, who present the highest level of skill and expertise, for what should be recognized as valuable contributions to the public discussion. Dr. Marik has received a commendation from the Virginia State Assembly precisely for his work in COVID-19 (Exhibit B). Dr. Marik was also a senior editor on the only published textbook for the treatment of COVID-19. (Exhibit C). The NIH heard directly from both Dr. Kory and Dr. Marik about ivermectin and COVID-19, and for a time, altered their ivermectin recommendation on their input; a clear recognition of their national expertise in this matter. As recognized experts at the forefront of research in the field, the proposed use of sanctions to shape debate on appears punitive and an inappropriate effort to shape public policy by declaring opinions ABIM disfavors as “disinformation.” Such a step would be an abuse of the Board’s discretion that would constitute a novel exercise and a misstep for the Board to undertake as a matter of law and policy.
I. Use by ABIM of Sanctions Based on its Own Independent Judgment about Disinformation is a Novel and Original Action that Requires Care as to Procedure; Consideration Must be Given of its Impact on the Development of Medicine; Further We Preserve Objections Regarding Standard of Care and Scope of the Inquiry.
Prior to the pandemic, the Board’s notices of proposed disciplinary action primarily involved disciplinary matters that had been reported to the National Practitioner Databank. There was no ambiguity; the question before the Credentials and Certification Committee (“CCC”), in such cases is whether the findings made by others rose to the level to which the Board should take action. The notices to Dr. Kory and Dr. Marik are altogether different; they propose to conduct original investigations rather than determine if administrative authorities or civil court findings, in which due process protections have already been observed, rise to the level of disciplinary action by the Board. The Board enters into this as an original adjudication in the highly complex public health arena of a novel pandemic about which there is a wealth of conflicting data, ongoing developments, and numerous conflicts of interest.
From our correspondence prior to submission of this statement, the Board does not appear to recognize that due process requirements for an original determination are altogether different than the Board’s usual and customary cases. We are also concerned from recent decisions that the Board does not recognize it is adjudicating standard of care determinations that are not ripe. The data is complex and ongoing and a scientific consensus cannot have yet fully emerged; we have asked the Board to state the standards and scope of its inquiry before requiring a response but the Board was unwilling to do so and we preserve those objections in this filing. The Board also does not appear to recognize that it is stepping into the regulation of professional speech, rather than practice, which would set poor Board policy as well as concerning precedent and be subject to legal challenge.
ABIM appears to poised to rely solely on the position taken by some public health agencies and organizations that any statements contrary to their interpretation of the evidence are dangerous. The efforts to label contrary views as “disinformation” are only as valid as the underlying view to which Dr. Kory and Dr. Marik have mounted a well-researched challenge. To merely hold that because public health agencies declare such opinions dangerous to public health and are thus off-limits, and refrain from properly considering the matter we submit as a result, would be circular; the assumption that the public must not hear divergent voices to improve compliance cannot be used to insulate questions about the underlying validity of those recommendations.
II. The Standard of Review Should be Clearly Articulated as Unsupported By Evidence Rather than Merely Disagreement with Public Health Messaging or Majoritarian Viewpoints.
When legitimate debate is at risk of being cast aside in an effort to enforce the current “consensus” viewpoint, ABIM must ensure that it makes an actual inquiry into the medical evidence rather than merely adjudicating whether a physician’s statements conflict with governmental public health agency messaging. The public narrative that ivermectin is a dangerous drug, for example, which would have been shocking to any physician prior to COVID-19, grew from false news stories and a broad reading of narrow public health agency concerns that people outside of a physician’s care were taking risks by using self-prescribed, most often veterinary, ivermectin without medical advice. As shown in this Statement, the ABIM process likely misunderstands the FDA position, which is silent on physician use of ivermectin in COVID-19, though as we have not had responses to our inquiries we have no notice of the standards and concerns pursued by ABIM. Specifically:
With regard to ivermectin and other repurposed drugs: We are not on notice of whether the allegations are based upon the novel view that diplomates must adhere to public health messaging without regard to the scientific evidence and of continuing health agency retreats from their positions and growing evidence of the defects in these policies. In simple terms, will the Board simply introduce an FDA, CDC or other policy statement it reads as contrary to Dr. Kory’s or Dr. Marik’s views and call it a day? Is the attached exercise in which we provide voluminous support for their statements futile, no matter their accuracy? Does the Notice purport to fault my clients for propounding bad science or for the mere fact of disagreement with public health agency policy? This is fundamental and the Board should have clearly articulated whether it has prejudged the matter by stating the nature of its allegation in the May 26, 2022 Notice of Potential Disciplinary Action (“Notice”). Our hope is that it will in fact afford a professional review of the evidence that comports with the scientific method and procedural due process.
The NIH is the only agency that has reviewed research and issued guidelines, and it has been careful to point out that its guidelines are just that and expressly says that they should not be considered mandates. The choice of what to do or not to do for an individual patient is ultimately decided by the patient and their provider.”[2] If the Board were to find against Dr. Kory or Dr. Marik based on the NIH Guidelines they would do so in direct contravention of that Guideline.
With regard to vaccination: The ABIM position statement is that it is disinformation to contradict the statement that vaccines are “safe and effective.”[3] It is unclear if ABIM’s position is that mRNA vaccines present no significant risk whatsoever and therefore diplomates may are not allowed to point to the plethora of research that shows specific risks; by usual standards of risk/benefit analysis and informed consent, such concerns are proper professional and public debate. The phrase “safe and effective” is a term of art meaning that a regulatory agency has concluded that the benefits outweigh the risks. It would be highly irregular to interpret that finding to mean that patients should therefore not be informed of the risks and that the evolving understanding of risk/benefit properly leads to a call to defer vaccination, particularly for specific patient groups such as children and young adults.
Vaccines appeared to present a case for a publicity campaign aimed at achieving herd levels of vaccination, yet as noted in the this Statement the CDC has acknowledged, and the evidence supports, little efficacy in reducing transmission. This places the point of decision on the extent to which it is preventing illness and reducing severity. While more controversial, the view that emerging data shows the risk/benefit point has shifted against use in many populations, such as the young or those post-infection, is well-supported by the evidence. Physicians have an ethical obligation to address the emerging data that is superior to any obligation to follow public health efforts to maintain its vaccination campaign across all populations at all costs. The data in this Statement makes clear that their positions are reasonable when measured by the data rather than the caricature painted by the harsh campaign against any dissent. The suggestion by ABIM that it would place agreement with public health narratives as a superior requirement to raising the contradictory medical evidence would be a novel, unsupported, and highly questionable precedent for a professional board certification body to take.
We acknowledge that the Board is rightly concerned about misunderstanding encouraging vaccine hesitancy. But even a casual read of the level of evidence and analysis contained in this Statement demonstrates that Dr. Kory and Dr. Marik hold well-researched views. On the topics of repurposed drugs such as ivermectin and concerns about mRNA vaccination, the level of data and analysis presented in this Statement is substantial and far beyond the level of evidence ordinarily required to set standards of practice. But whether, given ongoing changes in the evolution of the pandemic and its treatment, these matters have reached the maturity needed to set an enforceable standard of care is highly questionable. We question any presumption that there is a “standard of care” for treatment of a novel, rapidly evolving, unusually complex disease. “Standards of care” in response to the pandemic have, not surprisingly, continually changed.
In sum: On the issues presented, the Board must declare its standard and sources of authority so that the public and any appeals process can fairly judge its action; it either would ground such a decision upon mere disagreement with public health messaging or it must provide a proper and detailed review of the evidence provided in this Statement and explain why reliance on this information constitutes “disinformation.”
III. Any Adverse Decision Should Detail the Nature of the Evidence Presented Rather than Merely state Disagreement with Public Health or other Medical “Authorities”; The ABIM Should Remain Independent and Allow Diverse Opinion.
Given the foregoing, unless ABIM wishes to concede it is merely acting as a public health functionary and disciplining physicians for contrary statements without regard to the reasonable support for their views, were the ABIM to impose a sanction we would reasonably expect a substantial review and comment on the attached materials rather than a simple rejection based on differences with public health agencies and medical authorities, whether perceived or real. If the Board properly considers the scientific evidence submitted in this Statement and nonetheless recommends that Dr. Kory and Dr. Marik be subject to some sanction, we anticipate that the Board will have developed a detailed record showing it considered substantial competent evidence that contradicts our Statement to such an extent that no reasonable physician could hold these views, list the evidence that it relied upon, and not merely rest on its interpretations on “authoritative” public health agency statements for which we detail the basis for disagreement.
Given the immense economic and social disruptions caused by the pandemic, the government and public health agencies had an enormous incentive to maintain messaging that the vaccine was not only safe and effective but free of concerns to provide sufficient comfort for people to return to work. While reasonable physicians can look at the evidence differently, we think it important that ABIM allow, if not encourage, such diverse opinion.
IV. Avoiding Arbitrary, Capricious, and Inconsistent Determinations.
As but one example of the treacherous waters the ABIM is attempting to navigate, one of the sources of concern about excess mortality that may result from mRNA vaccination, a claim in the Notice to Dr. Marik, is its support in an article by Florida Surgeon General Joe Ladapo, M.D. “Exploring the relationship between all-cause and cardiac-related mortality following COVID-19 vaccination or infection in Florida residents: a self-controlled case series study.[4] As a result of this and other information about adverse effects, Ladapo recommended “against males aged 18 to 39 from receiving mRNA Covid-19 vaccines,”[5] essentially going against the recommendations of the Centers for Disease Control and Prevention (CDC) and numerous other scientific organizations around the world. When the FDA took the extraordinary step of approving mRNA vaccination for infants, Surgeon General Ladapo firmly came out against this step: “Ladapo opposes COVID vaccines for children younger than 5.”[6]
In the matter of Peter McCollough, M.D., the ABIM determined that it was a chargeable offense to state that “there is ... no scientific rationale ... for healthy people under 50 to receive a Covid vaccine.” Yet this similar policy is official policy in Florida by determination of its Surgeon General.
Surgeon General Ladapo is an ABIM Diplomate. This raises the question of whether, should the Board rule against Dr. Marik on this basis, the Board intends to take action against Dr. Ladapo’s diplomate status? If not, how would the Board justify maintaining this charge against Dr. Kory and Dr. Marik but not taking action against Surgeon General Ladapo? And how would it apply public health agency policy as an evidentiary guide in the face of disagreement between agencies? If either Dr. Kory or Dr. Marik is sanctioned because of statements that are supported by Dr. Ladapo we would expect similar action to be taken against Dr. Ladapo or a credible explanation that demonstrates that the Board’s actions are not arbitrary and capricious.
V. A Note about Legal Restrictions on Speech and Other Legal Concerns.
That ABIM has challenged Dr. Kory and Dr. Marik for bringing information based on a depth of expertise and literature review (only partially set forth in this Statement in the interests of everyone’s time) raises a host of legal issues. While we understand there are valid concerns about scientifically unfounded and in some cases politically motivated challenges to vaccination, an effort to restrict speech based upon an exploration of the literature by highly qualified physicians and discussion about those concerns with the public is a dangerous and concerning step for the Board to consider.
While we are aware that court challenges to the Board have largely been unavailing as ABIM is generally held not to be acting under the color of state law, using adverse actions against diplomate status as a tool to enforce compliance with public health agencies is a different matter than has been presented to the courts. If the Board were to sanction Dr. Kory or Dr. Marik merely because their statements are seen as inconsistent with CDC, FDA, or other governmental authority, that would not only be a policy that rejects ABIM as a home for learned debate but would arguably be an action to suppress speech under the color of state law. Further, we would consider any sanction that does not provide a response to the detailed material we have provided but simply rests on public agency positions to be arbitrary and capricious and a violation of my clients’ due process rights.
Given the hope that the CCC will be interested in reviewing and giving due consideration to the evidence in support of Dr. Kory’s and Dr. Mariks’ statements, we have focused our limited time on the scientific evidence rather than make a legal argument here. In the event of an adverse decision and the need to appeal, we reserve the right to further address the legal concerns we have with the Board’s approach, particularly as we have attempted to address this as a deficit in the original Notice without success.
Section Concluding Thoughts
It is not necessary that ABIM agree that ivermectin is an important drug in managing COVID-19 or with concerns about vaccination, only that the Board recognize that there is reasonable basis for these views and there thus no basis for imposing a sanction. ABIM can speak for itself and take issue with the statements that concern it without using its authority to squelch learned debate about these critical topics.
Section 2
The Work of the FLCCC and Respondents’ Credentials and Expertise
The ABIM Notice intimates that the work of the Front Line COVID-19 Critical Care Alliance (“FLCCC”) is suspect because its conclusions differ from consensus, a consensus that must be recognized is driven, in part, by conflicts of interest given the substantial pharmaceutical interests involved and the need to take actions that would calm economic instability.
FLCCC was founded by a group of highly published, world-renowned Critical Care physicians and scholars, including Dr. Kory and Dr. Marik, who have held leadership positions in large medical center ICUs. Its MATH+ Hospital Treatment Protocol was introduced in March 2020 and has saved tens of thousands of patients who were critically ill with COVID-19. (Exhibit D). The expertise in clinical research can be seen just in the fact FLCCC member physicians have nearly 2,000 published peer-reviewed publications among them. These eminent, well-recognized physicians have extensive experience with COVID-19, and, despite being overtime at bedside throughout this emergency, have put remarkable efforts into studying, documenting, and educating the professions and the public about the clinical value of ivermectin in COVID-19.
One of FLCCC’s initial efforts, consistent with WHO guidelines, was to explore the re-purposing of existing drugs, an effort that received too little global effort as financial resources focused on developing new patented medications. A rapidly growing published medical evidence base demonstrating ivermectin’s unique and highly potent ability to inhibit SARS-CoV-2 replication and to suppress inflammation included not only multiple in-vitro and animal models, but numerous clinical trials from centers and countries around the world showing repeated, consistent, large magnitude improvements in clinical outcomes when ivermectin is used, not only as a prophylactic agent, but also in mild and moderate cases and even has some positive effects even in severe disease states. FLCCC developed consensus-based standards among its global physician members, issued them for use by interested medical professionals worldwide, and advocated for their adoption and public discussion by physicians who recognize the need to inform the public about the value and availability of ivermectin. The Alliance has the academic support of allied physicians from around the world to research and develop lifesaving protocols for the prevention and treatment of COVID-19 in all stages of illness. The website cites a large number of peer-reviewed publications, some of which were authored by FLCCC’s founding physicians.
FLCCC, Dr. Kory and Dr. Marik have extensive international support, as evidenced by a letter directed to ABIM and signed by over a thousand physicians and other individuals alarmed by the use of diplomate sanctions to stifle well-reasoned and supported views simply because they contradict a questionable public health narrative.[7]
Pierre Kory, MD, MPA Credentials
Dr. Kory’s is Board Certified in Internal Medicine (currently), Pulmonary Diseases, and Critical Care Medicine and is a former Associate Professor and Chief of the Critical Care Service at the University of Wisconsin. To date, Dr. Kory has published over 50 peer-reviewed papers, 17 book chapters, and served as senior editor of an award-winning textbook now published in its 2nd edition and translated into 7 languages. He is currently the founder and Medical Director of a private telehealth practice opened 8 months ago called the Advanced Covid-19 Care Center (drpierrekory.com), which is solely focused on treating patients with COVID and its complications including “long haul” and post-COVID-mRNA vaccine injury syndromes. Most pertinently, he has published over 12 research papers on numerous aspects of COVID-19.) Dr. Kory has never had any malpractice claims or patient complaints. CV attached as Exhibit E.
Paul Marik, MD Credentials
Dr. Marik has special knowledge and training in a diverse set of medical fields, with specific training in Internal Medicine, Critical Care, Neurocritical Care, Pharmacology, Anesthesia, Nutrition, and Tropical Medicine and Hygiene. Dr. Marik has written over 500 peer-reviewed journal articles, 80 book chapters, authored four critical care books, and he is the senior editor of the only published textbook on COVID-19.[8] (Book Cover, Exhibit C.) He has been cited over 43,000 times in peer-reviewed publications and has an H-index of 77. He has delivered over 350 lectures at international conferences and visiting professorships. He has received numerous teaching awards, including the National Teacher of the Year award by the American College of Physicians in 2017. He is the second most published critical care physician in the world ever, and is a world-renowned expert in the management of sepsis. His contributions to the understanding and management of the hemodynamic, fluid, nutritional, and supportive care practices in sepsis have transformed the care of patients throughout the world. He also led the Society of Critical Care Medicine task force on corticosteroids in sepsis. He has already co-authored 18 papers on many therapeutic aspects of COVID-19. Dr. Marik;s CV is attached as Exhibit F.
It should also be noted that in his entire career in critical care, a highly litigious field, Dr. Marik has only received one complaint and has never been sued for malpractice. Further, Dr. Marik received a BIPARTISAN commendation from the Virginia Legislature for the work he has done with COVID (Exhibits B and G.)
Further, as noted in the body of this statement, the NIH has recognized both Dr. Kory and Dr. Marik are experts in the field and the value of their contributions around the questions of ivermectin in COVID-19.
Political Affiliations
Given the extent to which this issue has been politicized, presumptions that contrary views must be based on some form of radicalization, and extensive misinformation on the World Wide Web, it should be emphatically noted that FLCCC is a non-partisan organization and the politics of Dr. Kory and Dr. Marik cannot be deduced from their scientific positions, which should be judged solely on their scientific merits.
Ivermectin has been the subject of extensive misinformation in which the scientific data supporting its safety and effectiveness as an early intervention in COVID-19 has been clouded by a perfect storm of political controversy, in every sense of that phrase. It has become a hot button for critics who have hijacked the assessment of the science on both sides of the COVID-19 policy divide. Ivermectin has become a proxy in the debate over pandemic measures, used without understanding as a synonym for non-scientific thinking, an irony to the community of physicians who examined the evidence and saw its utility in actual practice.
[1] http://drelef.org/2022-open-letter-fsmb-abms/
[2] https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/guidelines-development/?utm_source=site&utm_medium=home&utm_campaign=highlights
[3] https://www.abim.org/media-center/press-releases/joint-statement-on-dissemination-of-misinformation/
[4] https://floridahealthcovid19.gov/wp-content/uploads/2022/10/20221007-guidance-mrna-covid19-vaccines-analysis.pdf?utm_medium=email&utm_source=govdelivery.
[5] https://www.floridahealth.gov/newsroom/2022/10/20220512-guidance-mrna-covid19-vaccine.pr.html
[6] See also: http://ww11.doh.state.fl.us/comm/_partners/covid19_report_archive/press-release-assets/g2-jtr_QWBT4hJpqr_20220308-1923.pdf
[7] http://drelef.org/2022-open-letter-fsmb-abms/
[8] Varon, J. Marik, P. Iglesias, J. de Souza, C. Challenges in the Pandemic: A Multi-disciplinary Approach. Thieme Publishing, www.thieme.com. (285 pages) 2022.
Section 3
Recommendations Made about the Use of Ivermectin in COVID-19
are Evidence-Based and Appropriate
I. There is Substantial Evidence That Ivermectin Has Significant Clinical Utility in Treating COVID-19.
A. Peer-reviewed, published evidence supports the use of ivermectin in COVID-19.
While the scientific evaluation of ivermectin for COVID-19 continues, there has been a clearly inaccurate narrative based upon the objectively false statement that there is no evidence to support the use of ivermectin in COVID-19. While there can be disagreement over whether the totality of evidence favors or disfavors use, there is a substantial body of evidence–far larger than that required to obtain new drug approval–supporting this indication. The oft-repeated drumbeat that “there are no scientific studies that show that ivermectin is safe or effective in the treatment of COVID-19" is contradicted by a substantial body of completed research including peer-reviewed meta-analyses. Presently, there are over 93 trials including at least 43 randomized controlled trials, which cumulatively show significant benefit. The studies are summarized at an extensive repository of studies listed at https://c19ivm.org[1] and a meta-analysis found at https://c19ivm.org/meta.html[2] (constantly updated), which are all incorporated into this response.
Over 133,842 patients have been included as study subjects with the overall signal of benefit in important clinical outcomes strongly positive with tight confidence intervals. A review of this evidence authored by FLCCC physicians is attached as Exhibit G.[3] This review was done using the Cochrane Risk of Bias 2.0 tool and the consistency of benefits seen from sets of randomized and observational controlled trials lend even more credibility to the estimates of benefit.[4] [5] While there is certainly misunderstanding and controversy around the issue, policy positions that refuse to recognize and fail to dispute this evidence are not legitimate.
Given the broad range of data available about ivermectin, analyzing its efficacy has been complex and required considering clinical studies with a wide range of designs and risks of bias along with a large body of epidemiologic data. Nevertheless, evidence for the effectiveness of using ivermectin for COVID-19 is found in numerous published, peer-reviewed meta-studies, clinical studies, epidemiological evidence, and clinical experience.[6]
One meta-study, performed by the FLCCC, assessed available studies using the Cochrane Risk of Bias 2.0 tool to assesses trial biases with the grades of “some concern, low, moderate, high, or serious.” Although one group of authors assessed many of the trials as having moderate to severe risks of bias, the meta-analyses of these trials by top scientists (including some affiliated with the WHO and other health organizations) enabled a more accurate assessment of the drug’s true effects despite individual trial biases. All found consistent benefits amongst the trials. In fact, the consistency of trial results–from sets of randomized and observational controlled trials from varied centers and countries and trial sizes and disease phases–lend even more credibility to the estimates of benefit.[7]
Recent publications of note include a systematic review and meta-analysis by Bryant and Lawrie,[8] which found clinically significant reduction in the risk of death and moderate evidence of substantial reductions in illness.[9] Summary analyses of the data from these trials find large, statistically significant reductions in time to clinical recovery, time to viral clearance, hospitalizations, and death as seen on the right of the below graphic A graphic tells the story at a glance:
https://c19ivm.org/
[2] https://c19ivm.org/meta.html
[3] Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Pierre Kory, MD, G. Umberto Meduri, MD2, Jose Iglesias, DO, Joseph Varon, MD, Keith Berkowitz, MD, Howard Kornfeld, MD, Eivind Vinjevoll, MD, Scott Mitchell, MBChB, Fred Wagshul, MD, Paul E. Marik, MD. Exhibit B. Note the publication include 5 pages of references to peer-reviewed literature.
[4] https://covid19criticalcare.com/treatment-protocols/totality-of-evidence/
[5] For e.g., Ivermectin Scientific Studies. Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial. https://www.medrxiv.org/content/10.1101/2021.05.31.21258081v1.
[6] The BIRD Recommendation on the Use of Ivermectin for Covid-19: Executive Summary, British Ivermectin Recommendation Development, Proceedings and conclusions of the British Ivermectin Recommendation Development meeting held on the 20th of February 2021 in Bath, United Kingdom. https://www.francesoir.fr/sites/francesoir/files/media-icons/bird-proceedings-02-03-2021-v151.pdf. See also https://bird-group.org/meta-analysis-paper/. BIRD is an international is a non-profit organization based in the United Kingdom advocating for Ivermectin and other safe established medicines and supplements to be used to prevent and treat covid around the world. For more information see
https://bird-group.org/
.
[7] https://covid19criticalcare.com/ivermectin-in-covid-19/.
[8] Bryant A, Lawrie T et al. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am. J. Therapeutics 0, e1-e27 1075-2765 (2021) https://covid19criticalcare.com/wp-content/uploads/2021/06/Ivermectin_for_Prevention_and_Treatment_of.98040.pdf
[9] See other meta-studies, including:
Roman YM, Burela PA, Pasupuleti V, Piscoya A, Vidal JE, Hernandez AV. Ivermectin for the Treatment of Coronavirus Disease 2019: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin Infect Dis. 2022 Mar 23;74(6):1022-1029. doi: 10.1093/cid/ciab591. PMID: 34181716; PMCID: PMC8394824.
Zein AFMZ, Sulistiyana CS, Raffaelo WM, Pranata R. Ivermectin and mortality in patients with COVID-19: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. Diabetes Metab Syndr. 2021 Jul-Aug;15(4):102186. doi: 10.1016/j.dsx.2021.102186. Epub 2021 Jun 27. PMID: 34237554; PMCID: PMC8236126.
Kory, Pierre MD; Meduri, Gianfranco Umberto MD; Varon, Joseph MD; Iglesias, Jose DO; Marik, Paul E. MD Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, American Journal of Therapeutics: May/June 2021 - Volume 28 - Issue 3 - p e299-e318 doi: 10.1097/MJT.0000000000001377
Ivermectin for COVID-19: real-time meta-analysis of 81 studies Covid Analysis, Apr 2, 2022, Version 184 [Together Trial analysis, Strongyloides, BBC, GMK]
https://ivmmeta.com
(Last accessed at 4/4/2022). Summaries and full text.
Figure 1. COVID-19 Ivermectin Studies (Source: c19ivm.org).
B. Epidemiologic Evidence Provides Further Strong Support.
In addition to these trials, the epidemiologic data presented by FLCCC may provide the strongest level of medical evidence attainable, as they consist of findings from what should be considered large, real-world “natural experiments” that spontaneously occurred within many cities and regions of the world when local and regional health ministries decided to initiate widespread ivermectin distribution to their citizen populations. Data that arose when various regional health ministries and governmental authorities within South American countries initiated “ivermectin distribution” campaigns–revealed temporally associated decreases in case counts and case fatality rates. The “control groups” in these natural experiments were the neighboring cities and regions that did not employ widespread ivermectin distribution. In the areas with ivermectin use compared to those without, large and temporally associated decreases in case counts and fatalities were found after the ivermectin distribution began. The magnitude and reproducibility from city to city, region to region, and country to country is unassailable. All data were sourced from universally used, publicly available COVID-19 epidemiologic databases. The FLCCC website contains extensive explanation and citation to the evidence supporting the use of ivermectin in all stages of COVID-19 and provides responses to critiques of that evidence.
All data were sourced from universally used, publicly available COVID-19 epidemiologic databases. The manuscript by Chamie et al, which focuses solely on this data has been refined and reviewed by scientists and researchers under the direction of a dean at a major medical research university. A number of these scientist researchers have joined as co-authors of this historically important manuscript.
C. Ivermectin as part of a Protocol Has Been Shown to Have Effectiveness as a COVID-19 Prophylactic.
Note that some of the 93 studies that have been completed on ivermectin for COVID-19 include studies that have found that ivermectin can play an important role in prophylaxis.
See also Review Article attached as Exhibit H (Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, Pierre Kory, MD, G. Umberto Meduri, MD2, Jose Iglesias, DO, Joseph Varon, MD, Keith Berkowitz, MD, Howard Kornfeld, MD, Eivind Vinjevoll, MD, Scott Mitchell, MBChB, Fred Wagshul, MD, Paul E. Marik, MD). Note the publication includes 5 pages of references to peer-reviewed literature.
D. FLCCC, Dr. Kory and Dr. Marik have emphasized that ivermectin is most effective as part of a protocol.
Ivermectin is generally given as part of a protocol with clinical synergies; isolating it leads to improper guidance. FLCCC, Dr. Kory and Dr. Marik recommend the use of ivermectin as part of a comprehensive protocol meant to work synergistically. Ivermectin is one of a number of evidence-based interventions but is generally not recommended alone. The protocol is designed to work by combining the clinical impacts of a number of different products rather than relying on single ingredients. Particularly given the complexity of COVID-19, multiple vectors including antiviral, anti-inflammatory, immune enhancing and others need to work in combination. Isolating ivermectin by itself and not recognizing the value of the entire protocol leads to poor medical decision-making and a determination divorced from the facts of the case.
II. Contrary Views are Based in Part on Misunderstanding Public Agency Positions about Safety and Efficacy, Unfounded Criticisms, and a Bias against Repurposed Drugs.
A. The FDA is incorrectly perceived as stating that physician prescribing Ivermectin for COVID-19 is improper.
While the ABIM notice is defective because it does not state any basis for its opposition to Dr. Kory’s or Dr. Marik’s statements to which we can focus a proper response, one of the frequent sources of baseless criticism is that ivermectin is not currently authorized or approved by the Food and Drug Administration for the prevention or treatment of COVID-19. While ABIM is operating from a presumption that ivermectin does not have an appropriate use in COVID-19, this appears to reflect a highly transmissible narrative and is note based on the FDA's actual position. Public perception on the use of ivermectin in COVID-19 has arisen in an echo chamber of agency positions targeting consumer-self-medication with ivermectin along with the challenge of evolving data. As ivermectin is a repurposed use of an approved generic drug, there is no financially feasible means to bring the issue before the FDA. The FDA has never considered the question. FDA, along with the NIH and CDC positions, arose when public health agencies became concerned about self-medication with veterinary or Internet products and that such use may interfere with public efforts for vaccination.
The following five statements conclusively demonstrate that FDA cannot be cited as supporting a view that ivermectin’s use in COVID-19 is below the standard of care. FDA has itself disclaimed the view in litigation that it opposes the use of ivermectin in COVID-19, and were the ABIM to use FDA’s pronouncements in its public campaign against self-medication with animal drugs it would be entirely without basis:
ds
1) The FDA does not set standards of care for the off-label use of drugs.
The FDA does not set standards of care. That would directly interfere in the state regulation of medical practice. See Chaney v. Heckler, 718 F.2d 1174, 1179 (D.C.Cir. 1983) (“FDCA’s legislative history expresses a specific intent to prohibit FDA from regulating physicians’ practice of medicine.”) rev’d on other grounds, 470 U.S. 821 (1985). What is commonly called FDA’s “practice of medicine exception” developed from Congress “not want[ing] to interfere with physicians’ treatment of their patients.” U.S. v. Algon, 879 F.2d 1154 (3d.Cir. 1989).
2) “Off-label” use of drugs does not indicate failure to meet standard of care.
The fact that a specific indication has not been approved does not mean such use violates the standard of care. As the FDA cannot dictate standards in medicine, it does not comment on off-label uses. While ABIM is certainly aware of this, much of the criticism has been that this use of ivermectin is off-label which carries no negative connotation. The most conservative citation is that 20% of drugs are prescribed “off-label.” Allowing such use is particularly important where, as with COVID-19, the only approved drugs were authorized using abbreviated methods, have high risk profiles, and have not been sufficiently studied to become a gold standard against which to judge treatment. At the time, the only approved drug was Remdesivir. To compare safety: there have been 420 U.S. deaths attributed to ivermectin over a 20-year period, while there have been 2,014 deaths attributed to Remdesivir though it was only approved by FDA on October 22, 2020 and given to far fewer patients. Remdesivir, which is considered the “standard of care,” was approved contrary to WHO recommendations against its use and a significant body of literature finding its risks outweigh any benefit. This is particularly the case when no approved drugs exist or have yet been sufficiently studied to become a standard of care against which to judge outcomes.
3) The FDA’s “You are not a horse” and other campaigns did not state an FDA position against such prescribing.
The FDA launched a media campaign directed at veterinary and other forms of self-prescribing, yet its “You Are Not a Horse” campaign was taken as a directive that physicians should not prescribe it. The source for FDA’s position comes from a consumer-facing page that was developed in response to a concern my client shares for the use of veterinary forms, as well as other allegedly reported safety concerns addressed below.
4) The FDA has not studied the use of ivermectin in COVID-19 and reached no conclusion that its use in COVID-19 is not safe or effective.
FDA took it upon itself to issue a broadly phased warning, but until a short time ago the FDA page acknowledged that “FDA has not reviewed data to support use of ivermectin in COVID-19 patients to treat or to prevent COVID-19..” While this statement has been removed, it remains true. The FDA has never studied the treatment of COVID-19 with ivermectin. The sole FDA position, on a consumer-facing page addressing self-prescribing, did not purport to guide physicians. The FDA has not made that statement to physicians, nor could it, as the FDA has never conducted any review of the safety or efficacy of ivermectin to treat COVID-19. The Agency could not meaningfully or lawfully issue such a statement given the lack of formal review or publication of any guidance noticed for comment about ivermectin’s use.
5) The FDA has expressly disavowed any intention to set the standard of care for the use of ivermectin in COVID-19.
The FDA in fact disavowed this unlawful view in litigation.As a result, the FDA disavowed the position that physicians may not prescribe ivermectin. See Exhibit I, Transcript of oral argument dated November 1, 2022, notably at pg.5, “These statements included nonbinding recommendations to consumers who could purchase animal-use ivermectin over the counter not to take ivermectin to treat COVID-19, but the statements did not say that doctors could not prescribe ivermectin to treat COVID-19 or that consumers could not take ivermectin for that purpose.” (emphasis added.)
B. The Centers for Disease Control and the Facts about the Safety of Ivermectin.
Another public agency position that has engendered similar confusion is the health advisory issued by the Center for Disease Control and Prevention (“CDC”). Public health agency alarm over self-prescribing, particularly of veterinary forms of the drug, led the CDC as well as the FDA to issue safety alerts. These results were triggered by false reporting of hospitals being overrun with ivermectin patients and manipulated reporting of Poison Control Center calls, which fueled media attention about the alleged dangers of ivermectin, a drug has been prescribed nearly 4 billion times with an extremely high safety profile. Significantly, the CDC webpage cited in the Notice contains only two examples of patients who suffered adverse reactions from ivermectin. Neither of these cases were prescribed nor followed by a physician, one used an animal formulation of unknown strength, the other self-prescribed and obtained some version over the Internet. These cases offer no evidence whatsoever that ivermectin in the hands of a trained physician presents any unreasonable risk to their patients.
The alarms raised about potential for overdosing are not grounded in real concerns as ivermectin is one of the safest drugs known. Studies using ivermectin doses up to 10 times the FDA approved dose of 0.2mg/kg have not been associated with any increased adverse effects. Ivermectin is on the WHO’s list of essential medicines, has been given nearly 4 billion times around the globe and is widely considered a safe drug. According to the WHO, it is safer than both aspirin and Tylenol. Its discoverers were honored with the Nobel Prize in 2015 for the drug’s global and historic impacts in eradicating endemic parasitic infections in many parts of the world. The FDA’s concern about use of animal drugs is certainly legitimate given the lack of safety data around their use. There is good scientific evidence that the escalating doses required to maintain antiviral levels have been subjected to considerable testing and are in fact safe.
The risk profile for ivermectin has been driven by and in turn feeds a false media reporting; in New Mexico, for example, two people died from COVID-19 but officials falsely reported they died from ivermectin toxicity, perhaps making assumptions because of alarmist news stories that were circulating. This led the CDC to issue the alert given a few reports of severe illness associated with the lay use of ivermectin.
The use of the CDC alert to stop physician prescribing due to alleged toxicity is inconsistent with substantial evidence of safety. Citing a 24-fold increase in prescribing from the pre-pandemic baseline, the alert was issued because of a five-fold increase in calls to Poison Control Centers (the National Poison Control Data System) (“NPDS”). Most of these involved patients consuming large amounts of veterinary or unknown forms purchased on the Internet and taken without medical supervision.
This five-fold increase in NPDS calls to justify a ban is highly misleading. A more fulsome description might be that a 24-fold increase in prescribing resulted in only a five-fold increase in calls. To better understand the overall safety signal it is useful to look at absolute numbers in data from the FDA Adverse Events Reporting System (FAERS). While poison control calls and FAERS each suffer from limitations, it is notable that reports for products containing ivermectin actually fell slightly in 2020 and 2021, despite increased use and dosing, with a combined total of 503 adverse reports at an annual rate less than 2017-2019. Reports did not rise post-COVID-19, but actually fell. The media reports about deaths due to ivermectin use turned out to be faked, as did the story about clogged emergency rooms, and the two incidents cited on the CDC report raising the concern were particularly egregious cases of misuse that can occur with any medication, in these cases drinking an injectable form prepared for use in cattle and a case of unknown strength purchased on the Internet.
Analyzing state-by-state Poison Control Center reports supports the NPR reporting that over 70% of calls were based on self-prescribed animal drugs and that a significant portion of human drug reports involved interactions with other drugs, a universal issue in prescribing which is why it is important that prescribing should be available unhindered so that physician judgment and human drugs are available and consumers do not turn to unsupervised use.
Until ivermectin was suggested to play a major role in the management of COVID-19 in
competition with much more profitable drugs in the pipeline, it was widely considered to be an extremely safe drug. A rejection of advocating for physician prescribed ivermectin for COVID-19 on the basis of safety would need to explain the concern in the light of this figures.
While a proper analysis of this information isn’t readily available, it is useful to note that the NPDS report CDC on ivermectin adverse events cites 1,140 reported cases between January 1 and September 21 of 2021. If one extrapolates that 70% of these were consumers of the animal drug, then in that eight-month period there were 285 calls regarding human drugs, or about 11 per week. According to the CDC health alert, there were approximately 39,000 prescriptions per week at the beginning of 2021 and rising.
Poison control calls are not only a de minimus percent of that number but looking more closely at the NPDS report for ivermectin use in COVID-19, far less than 1% are listed as leading to death (though there is no such confirmed case information, and it is not clear the NPDS is in a position to distinguish COVID deaths and adverse events from medication). About 2% reported a major effect and 10% a moderate adverse effect. Even assuming these were validly ascribed numbers and extrapolating this 13% apparent effect size to all of the 1,440 reports, there were about 187 cases that reported some level of moderate to serious concern over a time frame in which over one million scripts were written for ivermectin with adverse events reported in roughly 0.019% of cases. Of those, the majority would be from animal forms, leaving an estimate of moderate to severe events with human drugs in the range of 1 in 40,000 prescriptions. This is a remarkable safety record.
C. The NIH Position Has Shifted Several Times and its Guidelines Do Not Support Discipline.
The only agency that has actually conducted an analysis of the data for ivermectin for use against COVID-19 is the National Institutes of Health (“NIH”). The NIH position has varied; originally they found there was insufficient evidence to recommend ivermectin, upgraded that recommendation to state that there is insufficient data to “either recommend for or against the use of ivermectin in COVID” and then later, based upon the TOGETHER and ACTIV6 trials, whose defects are discussed below, reverted to a position that there is insufficient evidence.
It is important to note that “neither recommend or deny” determination, which clearly leaves the decision up to the individual physician, was made after the scientific evidence supporting this use was personally presented directly to the NIH COVID-19 Panel by Dr. Kory and Dr. Marik. The NIH panel met in mid-January of 2021with Dr. Marik (at the time Chief of the Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA) and Dr. Kory (at the time, Chief of the Critical Care Service and Medical Director of the Trauma and Life Support Center at the University of Wisconsin) founding members of the FLCCC Alliance. After FLCCC’s presentation, the NIH immediately elevated ivermectin from its original “do not use” to a “neither recommend for or against” policy. This fact alone should make clear that both Dr. Kory and Dr. Marik are an active and respected part of the national debate over this treatment and the threat by the ABIM to take disicplinary action is an inappropriate imposition on that debate.
For a time, ivermectin shared the “neither for or against” NIH category with monoclonal antibodies and convalescent plasma for COVID-19. Both therapies had been commonly used in COVID-19 treatments without, to the best of our knowledge, any effort by ABIM to sanction physicians for employing such therapies. Particularly given the dearth of standard of care options in treating the novel coronavirus, such a neutral finding by the NIH historically places ivermectin’s use squarely within the reasonable judgment of the physician during that time.
Physicians since that reversal who are deeply and directly involved in the research and paying attention to the evidence certainly have a right to disagree. The basis for that disagreement is shared below and also in an FLCCC document. It has been uniformly recognized that available therapies for COVID are entirely inadequate, recent approvals notwithstanding, which places a different standard on physicians to make treatment choices and the freedom to speak and practice without interference.
Further, NIH Guidelines are just that, and NIH expressly says that they should not be considered mandates. The choice of what to do or not to do for an individual patient is ultimately decided by the patient and their provider.”(emphasis added.) It would therefore be inappropriate to rely upon the NIH guidance as a basis for enforcing a standard of care in a disciplinary proceeding.
D. The AMA position and other Echo Chambers Provide no Support for Action against Dr. Kory or Dr. Marik.
The American Medical Association (AMA), American Pharmacists Association (APHA), and American Society of Health-System Pharmacists (ASHP) have made statements on the issue but they have made no substantive determinations. The AMA, APHA, and ASHP are professional trade associations that have no review process and are not standard setting agencies. Their public statements serve merely to further echo the same erroneous narrative discussed in this Statement. They do not provide a basis for setting standards of care or determining what is disinformation.
Merck, who originally manufactured ivermectin, has also taken a position which certainly cannot be used either: at the time of its statement, Merck had a competitive drug to ivermectin in the pipeline; ivermectin is a generic that sells for approximately one dollar per pill and would bring Merck no revenue, whereas Molnupiravir sells for approximately $700 per course of treatment. Merck had enormous financial interest in feeding the narrative that ivermectin was not effective.
Merck claims that its statement was based on its review of the evidence. It does not state that it conducted any studies itself, but merely opines on its interpretation of available data. It is merely an opinion, biased for the reasons described, and does not add any new information to the question.
III. The Ivermectin Critiques Do Not Undercut its Demonstrated Value.
There are clearly substantial differences of professional opinion about the use of ivermectin. FLCCC has exhaustively reviewed and commented on the concerns that have been raised in its FAQ section and elsewhere. Addressing the central criticisms:
A. Published Metastudies Reaching Contrary Conclusions are not Well-founded.
One critique is based upon a metastudy reaching a contrary conclusion in a Cochrane Library Review. While similar methodology was used to that followed by reviewers who reached favorable conclusions, the main differences between these reviews are the criteria for selecting which studies should be included. The Popp Cochrane review only selected 14 of the 31 published studies available at the time, rejecting large studies with positive effects on questionable grounds, such as a demand that only studies with PCR testing be included even though availability and accuracy varied considerably, especially at the time; inconsistent rejection of comparators such as disallowing trials against hydroxychloroquine even though it has been determined by these same reviewers to without clinical effect and thus could properly serve as a control/comparator group; and the exclusion of combination therapies even though that is how it is actually used in practice. A principal criticism the Popp authors had of favorable studies was inclusion of those that used doxycycline in the intervention arm, complaining that the impacts of doxycycline could not be separately determined. Popp, ibid. at 32-33. While there may be some sense to this, given complications such as pneumonia, if doxycycline had a significant therapeutic impact on COVID-19 we would live in a better world.
In five of the included studies in the unfavorable Popp review, subjects only received a single dose, ibid, which could not have possibly reached therapeutic levels and are not valid studies. Subjects only received the FLCCC-recommended dosing in 5 of the 14 studies. Ibid. The study authors expressly state that they were aware of the dosing issue but did not have sufficient information to look at dose-response curves, ibid. at 13, yet included low-dose studies in the analysis in any event. Ibid. at 17.
Much more can be said, of course, about the science and the nature of these professional differences of opinion. The only reasonable conclusion is that that is what these are, i.e., legitimate professional differences of opinion. Physicians should be allowed to make their own determinations in the face of such differences without being subject to challenge, particularly in the context of a largely medically unresolved pandemic.
B. Criticism of Favorable Studies is not Well-Founded.
Other primary criticisms have been that many of the studies showing ivermectin’s effectiveness were small, poorly designed and executed, or with high risks of bias. As all clinical trials suffer from risks of bias in their design and conduct, as assessed by the Cochrane Risk of Bias 2.0 tool, performing meta-analyses can more accurately detect the true effects despite individual trial biases.
One real-time meta-analysis of dozens of studies of ivermectin shows statistically significant improvements for mortality, ventilation, ICU admission, hospitalization, disease progression, recovery, cases, and viral clearance. An early pooled analysis showed a 63% improvement for early treatment, 39% improvement for late treatment and 83% improvement for prophylaxis. In order to avoid a statistically significant result, the researchers say they need to exclude more than half of the studies, but the evidence, as summarized in Figure 1, has only become stronger.
C. Null Studies Purporting to No Results Have Substantial Defects.
Critiques of FLCCC, Dr. Kory's and Dr. Marik's client’s views, including NIH’s more recent position, primarily cite recent large, randomized controlled trials that have been represented as showing that ivermectin is not effective for COVID-19. Many of the trials have extreme conflicts of interest and appear to have been designed to fail and predetermined to show ivermectin as ineffective. Many studies, for example, use a monotherapy when the strong view of FLCCC, Dr. Kory and Dr. Marik is that ivermectin is most effective as part of a treatment protocol that includes other FDA-approved medications and supplements backed by clinical and observational evidence.
The trials often under-dosed and started treatment far too late, even though in the medical community it is common knowledge that COVID-19 becomes far more difficult to treat the longer a patient has had symptoms. Treating early is imperative.
The TOGETHER trial, for example, studied patients who started treatment up to eight days after the onset of symptoms. ACTIV-6 severely limited the use of ivermectin, administering a dose below what is known to be effective on the variants at the time and received too late (6 days on average) after the onset of symptoms. Despite these obvious shortfalls, in ACTIV-6 there was a statistically significant, albeit modest, impact on time to clinical recovery for patients using ivermectin to treat COVID-19. This effect was prominently seen in the more severe patients in the trial, whose symptoms were reduced by an average of three days with ivermectin. FLCCC physicians have understood for over 18 months that ivermectin works best against COVID-19 when administered early, in combination with other therapies, and given with a fatty meal for at least 5 days or until symptoms resolve.
This requiremeant for early intervention before the virus fully takes root is well-known and appears to be universally true for antiviral medications. This has been true for the flu, as with Tamiflu, and is also true of the two recent entries in the field by Merck and Pfizer. The pushback against use, largely by sources who do not evidence an awareness of the FLCCC body of literature, has made it difficult for patients to obtain ivermectin at the stage in which it is most useful. Critiques of ivermectin are often based on studies using interventions that were too late, too low in dosing, or on high-profile cases in which ICU patients weeks into the disease were finally given ivermectin, and unsurprisingly, it had less change of having an effect. Molnupiravir (recently approved from Merck) and Paxlovid (recently approved from Pfizer) would not fare well in such conditions either. We emphasize that, just as with the newer antivirals that have been approved, it is vital to have this treatment kit prepared and “on-hand” to take upon first symptoms of any viral syndrome like illness. The importance of early treatment can be seen in the graph below, showing diminishing efficacy of treatment with each day of delay. Note the near 100% efficacy if treatment is started within 24 hours of symptoms. All supporting data for this section can be found at c19early.com.
IV. Safety Comparisons with Other Treatment Options.
Other available medications have difficulties with effectiveness and certainly have more troubling safety records. One concerning approved drug is Remdesivir. The level of side effects in approved drugs is one of the reasons that the Nebraska Attorney General (Exhibit J) found that ivermectin prescribing was proper and his Opinion puts the ivermectin data into stark perspective by comparing them with far more numerous adverse events from Remdesivir’s use in COVID-19. Also note that the WHO found that Remdesivir was not safe and effective for COVID-19 and objected to its approval. Professional and regulatory judgments have differed strongly on all available potential therapies on these complex topics, making it a topic ill-suited to treat with such certainty as to not only deny patients access or to impose sanction when no treatment choice has reached sufficient clarity to develop a standard of care for the treatment of COVID-19 patients.
Paxlovid is contraindicated if a patient is taking a significant list of other drugs and has a higher risk. Since its approval in 2022 it has already had 21,249 adverse event reports to FAERS including disease reoccurrence, compared to 503 for the entire pandemic reported for ivermectin. As with many drugs advanced for COVID-19, confusion has been engendered by reporting retlative rather than absolute risk. Early results, for example, appeared to dramatically lower the combined outcome of COVID-19 related hospitalizations or death from any cause compared to the placebo group when expressed as a relative risk reduction of 88.9%; these results are less dramatic when they are expressed as an absolute risk reduction of 5.81%. A host of other criticisms about study data also suggest context in which favorable data need to be considered.
Molnupiravir has not shown high levels of effectiveness, shows 1,743 adverse events, and has not shown significant efficacy at reducing death rates. Studies are continually published showing poor safety and effectiveness, for example a recent study in Lancet showing that “Molnupiravir did not reduce the frequency of COVID-19-associated hospitalisations or death among high-risk vaccinated adults in the community.” While these drugs may have a role to play in treatment, a fair comparison shows that ivermectin is more effective and demonstrably safer than other available treatments and far safer than the one drug–Remdesivir–that the FDA initially approved for use against COVID.
V. The Validation of Repurposed Drugs Faces Nearly Insurmountable Real World Challenges.
A large body of excellent published scientific work has been ignored for unsound reasons. Public health agency concerns specific to the misuse of veterinary versions of the drug were amplified and misinterpreted. Financial incentives and regulatory blind spots that limited investigation into repurposing existing drugs made ivermectin a particular target for misinformation. There is little in place to provide an independent system dedicated to conducting well-designed trials and transparent research studies of repurposed generic treatments–not just for COVID-19, but for all diseases that may have safe and affordable remedies. The use of independent research is our only hope of understanding how these medicines can best be used to help patients.
The FDA’s role in treatment choices requires further comment here, as physicians working to reduce transmission and treating COVID patients had an utter lack of support from FDA given its sole attention on new medications and vaccination and a complete lack of attention to repurposed drugs. The FDA system of drug regulation provides limited avenues for approval for new indications of existing drugs and left physicians facing the pandemic with little guidance. As the FDA does not have reasonable mechanisms or regulatory pathways for the approval of repurposed drugs, it left physicians to make their own determinations based on available evidence. The NDA process is tailored to brand-new, patentable drugs and public health resources were focused on new drug and vaccine development and the scant attention paid to researching and validating the use of existing, often inexpensive drugs for COVID-19 was a major disservice. Public messaging targeting effective therapies that undercut expensive new drug development and vaccination was not tolerated. As a result, much of the data investigating ivermectin in COVID-19 was done overseas because the United States failed to devote resources to what inexpensive and widely available drugs could provide assistance, data which was then discounted in significant measure because it was done overseas.
This issue raises substantial public policy concerns given that repurposed drugs have not only been given short shrift as subjects of research but are actively opposed. Off-patent, generic, or over-the-counter therapies are not recommended in this country, despite often higher amounts of trials evidence for their use. Note that the grey font indicates medicines with less than 5 trials to support in the chart below.
As of May 2022 massive evidence bases supporting numerous generic, repurposed drugs with excellent safety profiles that act with either anti-viral, anti-inflammatory, or immunomodulatory properties have been compiled. The medicines shown effective can be seen below. Below are circled only those medicines that have received Emergency Use Authorization status by the FDA or recommended by the NIH. Note that these “officially approved” medicines consist solely of novel pharmaceutical industry products that can generate massive profits, an obvious feature of our health care system in the United States.
Section Conclusion Thoughts
The view that ivermectin is an important, safe, and cost-effective treatment for COVID-19 is based on a plethora of evidence that has been carefully examined by highly qualified physicians. The public campaigns against the use of ivermectin, whatever their motives, have been misunderstood as evidencing a determination that the treatment is not appropriate and below the standard of care. The FDA has emphatically asserted on court records that it did not make such a statement. The only agency that has actually conducted such a study is NIH, which recognized Dr. Kory and Dr. Marik as experts on this topic and for a time changed their view of ivermectin as a result of their research. In changing its recommendation to return to a “do not use” recommendation, the NIH relied on studies that Dr. Kory and Dr. Marik reasonably do not find credible for reasons described above. NIH explicitly states that its conclusions are merely guidelines and do not set the standard of care, and it would be inappropriate for ABIM to impose sanctions on dedicated physicians involved in the national discussion presenting this level of evidence and consideration for an important treatment that has been vilified and overlooked.
Section 4
Hydroxycholoroquine and other Repurposed Drugs
I. There is Sufficient Evidence to Recommend Hydroxychloroquine, Particularly When Viewed Absent to Political Tropes Surrounding its Use.
Hydroxychloroquine use in COVID-19 became highly politicized and became a trope to represent unscientific recommendations based on its political positioning rather than the science. Globally, hydroxychloroquine has 379 controlled trials which involve almost a half-million patients. The studies show consistent, reproducible reductions in the incidence of all outcomes, particularly when given early, similar to ivermectin.
Detail of these studies can be found at www.hcqmeta.com.
This results of these studies are further detailed below:
Section Concluding Thoughts
The recommendation, contrary to the public narrative, does have an evidence base in support. This was particularly important when there were no available treatment alternatives.
Section 5
Dr. Kory’s and Dr. Marik’s Statements about Vaccination are Evidence-Based, Entirely Appropriate, and not Disinformation
Brief Summary: There is Significant Evidence That the Contribution of mRNA Vaccines to Reduction in Transmission, Prevention and Severity and Duration of Illness Is Over-reported and that it Presents Significant Risks; A Summary of These Critical Issues
The statements regarding vaccination made by my clients are based upon an examination of evidence regarding the relative risks and benefits of vaccination, data about natural immunity in post-COVID-19 patients, excess mortality data, and other markers of effectiveness and safety.
Transmission: Current data do not support the claim that the COVID-19 mRNA vaccine is effective in preventing transmission. The CDC Director herself has reported that vaccinated individuals are now well known to carry equal or greater viral loads than the unvaccinated, and thus transmit at equal or higher rates, for physiologic reasons detailed below, most concerning being the negative efficacy of the vaccines against Omicron. This has also been reported by seminal nosocomial outbreak papers by Chau et al. (Health care workers (HCW) in Vietnam), the Finland hospital outbreak (spread among HCWs and patients), and the Israel hospital outbreak (spread among HCWs and patients).
A large new study from Qatar in the New England Journal of Medicine by Weil Cornell Medicine found that the Pfizer vaccine protection waned after four months. By seven months, when adjusted for those who already had prior infection, the Pfizer shot had a negative 4% effective against transmission. Also, effectiveness against asymptomatic infection was negative 33% after seven months, which suggests that the vaccinated become more likely to spread COVID-19 over time.
Prevention: The claim that COVID-19 mRNA vaccine is effective in preventing illness is also not well-supported by the data. Using up-to-date data (i.e. last 3-6 months to today) from a wide selection of public health sources including the U.S, Denmark, Israel, Australia, and the UK, the current estimate of the protective efficacy from contracting COVID-19 is one of either “negative efficacy” or rapidly waning efficacy such that potential benefits, if any, are demonstrably short-lived and instead rapidly transition into an increased risk (i.e. negative efficacy) of contracting COVID-19.
Preventing Severe Disease: Similarly, the efficacy of the COVID-19 mRNA vaccine in preventing severe disease is also questionable. CDC data shows that there is no statistically valid evidence that they prevent severe disease or deaths in children. See below corresponding section which more fully details the data supporting this conclusion
Preventing Long Haul COVID-19: Finally, the efficacy of the COVID-19 mRNA vaccine in the prevention of “long-haul” COVID-19 does not appear to be supported. A large Veterans Administration study recently reported disturbing evidence: by month six after a SARS-CoV-2 infection, vaccinated persons with breakthrough infections were at higher risk of long COVID-19 (HR = 1.50, 95% CI: 1.46, 1.54). When including the earlier time periods, the COVID-19 vaccines only reduced the risk of long COVID-19 by approximately 15% compared to the unvaccinated, a level of estimated protection far less than the increased risk of death found in the same study as mentioned above.
Significant Risk: As detailed in this Section, the risks of myocarditis, neurological conditions, sudden death, and other adverse effects directly related to the mRNA vaccine are significant. In addition to the many published studies noted here, the government’s own V-Safe and VAERS data raise significant concerns.
I. Clinical Evidence for mRNA Vaccine Risks Are Significant and Have Been Underplayed.
A. There is substantial data that “all-cause” mortality has been elevated by the mRNA vaccine.
There is ample evidence supporting concerns that mRNA vaccines bear some responsibility for excess mortality. In this published paper analyzing data from the pivotal clinical trials used to support the novel mRNA vaccines (i.e., Moderna, Pfizer, and Janssen), Classen compared “all-cause severe morbidity,” defined as “severe infections with COVID-19 and all other severe adverse events between the treatment arms and control arms, respectively.” His analysis found a statically significant increase in all-cause severe morbidity occurred in the vaccinated group compared to the placebo group.
A shift in the basis for excess mortality is also cause for concern. As a result of a FOIA application in the state of Massachusetts, an analysis of the now publicly available death certificate data found that during 2020, the predominant causes of rises in all-cause mortality were due to “respiratory causes,” (i.e. excess mortality from COVID-19) while in 2021, the predominant causes were “cardiovascular.” The analyst concluded, “the official Massachusetts database of death certificates contains proof that C19 vaccines killed thousands of people in Massachusetts in 2021.”
The CDC data shows the timing of the start and the steady rise in all-cause mortality of working-age adults in the U.S, both overlapping with the start of the mass vaccination campaign. Although alternate causes of this historic rise in death have been considered, (i.e. COVID-19 deaths, deaths of despair, etc.), the number of deaths from these causes is insufficient to explain the overall rise.
Florida Surgeon General Joe Ladapo published a study entitled “Exploring the relationship between all-cause and cardiac-related mortality following COVID-19 vaccination or infection in Florida residents: a self-controlled case series study.
There is extensive literature making these same points. In the following sections we explore the evidence and basis for the risks about which my clients have spoken.
B. V-Safe Data Shows Significant Safety Concerns that Public Health Authorities have not Communicated to the Public.
The CDC V-safe data show that 33.1% of the people who got the vaccine suffered from a significant adverse event and 7.7% had to seek urgent professional medical care. The CDC created this smartphone-based program to collect health assessments after COVID-19 vaccination. Approximately 10 million people signed up and submitted health reports after COVID-19 vaccination. Significantly, death is not a reportable category in V-safe as these are self-reports, and particularly given data about likely mortality from the vaccine set forth below, the 7.7% of respondents who sought urgent care is a concerning figure of which the public is largely unaware and the CDC evidently did not want to share because of its likely impact on compliance. The Informed Consent Action Network (“ICAN”) legal team sued the CDC twice; the CDC spent 463 days resisting ICAN’s efforts to obtain the data and make it public.
These extraordinary numbers clearly raise substantial questions about mRNA vaccine safety and the conduct of the CDC in resisting making this public data available to the American public is concerning. The contrast between this data and that reported in the clinical trials raises legitimate areas of inquiry.
ICAN has taken the CDC’s official raw data and created a dashboard interface that allows users to graphically view the 144+ million health entries. This data is based on self-reports of approximately 10 million V-safe users. Note that the data is limited to only pre-populated fields checked by V-Safe users (for example, selecting from a list of pre-populated symptoms). Information captured in free-form fields has not yet been released by CDC and litigation continues to obtain that information.
A Rasmussen Report using polling methods lines up closely with V-Safe, finding that 7% of vaccinated Americans have suffered major side effects from mRNA vaccine.
The CDC claims it took so long to release the data because they did not have enough resources to write the program to display the data, though plaintiffs were able to write that program in a day. It also does not explain why they opposed the release of data in court. The following figures taken from the ICAN Dashboard provide an overview of self-reported impacts, including the fact that of 10.1 million V-Safe users, over 751,000 required care, and of these, 73% felt the need to go to an urgent care center, ED or hospital.
C. VAERS Data Reveals Significant Safety Concerns.
As of May 27, 2022, in the United States alone 5,309 cases of myocarditis, 782,665 adverse events, 151,796 severe adverse events, and 14,613 deaths have been recorded in the Vaccine Adverse Event Reporting System following COVID-19 vaccination in the USA. It
should be appreciated that the VAERS database’s main limitation is that of underreporting, with a recent pre-print analysis suggesting AERS deaths are underreported by a factor of 20. The most concerning implication of under-reporting is in regard to the exponential increases in actual reports of death after vaccination in the past year compared to prior years of all vaccines combined.
Even more damning is the temporal relationship of these reports to the date of the individual’s vaccination, which some authorities have attempted to dismiss as simply representing “background” deaths. The fact that the reporting of deaths decrease over time from date of vaccination (shown below), infers a worrying causal relationship whereas erroneously
reported “background deaths” would instead appear in similar numbers each subsequent day after the date of vaccination.
Statisticians and analysts working with the Vaccine Safety Research Foundation (VSRF) have estimated the total number of deaths in the U.S caused by the COVID-19 vaccines based on the numbers reported to the U.S Vaccine Adverse Event Reporting System. In their white paper, they employed 9 different statistical prediction models and found that as of December 2021, total deaths associated with the vaccines ranged from 148,000 to 216,000. Using the same methodology for the 14,613 COVID-19 vaccine associated deaths in the U.S reported as of May 16, 2022, the updated point estimate is approximately 599,000 deaths. The data and conclusions from these publications above provide support for identifying the vaccination campaign as the primary cause of the massive increases in Life Insurance claims among working-age Americans beginning in the second half of 2021, as will be detailed below.
D. Epidemiologic Data Demonstrates Highly Alarming Safety Signals.
An article published in the journal Nature reported:
- increases of over 25% in the number of ambulance calls in response to cardiac arrests (CA) and acute coronary syndromes (ACS or “heart attacks”) for young people in the 16–39 age group during the COVID-19 vaccination rollout in Israel (January–May, 2021) compared with the same period of time in prior years (2019 and 2020).
- a robust and statistically significant association between the weekly CA and ACS call counts and the rates of 1st and 2nd vaccine doses administered to this age group. Note they found no observed statistically significant association between COVID-19 infection rates and the CA and ACS call counts.
- findings that aligned with previous studies showing that increases in overall CA incidence were not always associated with higher COVID-19 infections rates at a population level, and that the stability of hospitalization rates related to myocardial infarction throughout the initial COVID-19 wave compared to pre-pandemic baselines in Israel.
- findings that mirrored reports of increased emergency department visits with cardiovascular complaints during the vaccination rollout in Germany as well as increased EMS calls for cardiac incidents in Scotland.
Equally alarming is the massive rise in deaths among healthy, young professional athletes from around the world. Since the vaccination campaign was initiated, and as of June 4, 2022, there were approximately 1,616 athletes that suffered a cardiac arrest, with 1114 of them dying as a result. The majority of arrests occurred in competition or training. The frequency of these events in comparison to historical data is highly concerning. In a 2009 review of professional athletes deaths, published in a prominent European Cardiology journal, researchers found that from 1966 to 2004, there was an average of only 29 sudden athlete deaths per year worldwide. Compare this number to just the month of January 2022 alone where 127 collapses and 87 deaths among professional athletes were reported. Overall, these athlete deaths reflect an approximately 22-fold increase in the year after the introduction of COVID-19 vaccines, to date unexplained by other identifiable causes.
On February 10, 2020, the Israeli Health Ministry published the results of a survey of adverse events among roughly 2,000 random Israelis who received booster shots. Although many could be thought of as minor, it is concerning that 51% of the women and 35% of the men who experienced a side effect reported that, as a result, they had difficulty performing daily activities. A total of 4.5% of those who received booster doses reported neurological side effects.
E. There are substantial risks associated with receipt of a COVID-19 mRNA vaccination, particularly in younger patients, which have been globally recognized by a number of governments who are at odds with the CDC position.
Dr. Kory and Dr. Marik do not stand alone in expressing concerns, particularly among younger patients. Seven nations have suspended COVID-19 vaccines for younger age groups due to risks of myocarditis. Sweden, Finland, France, and Germany suspended Moderna for under 30 years old. Denmark suspended the Moderna vaccine for under 18 years old and now no longer recommends vaccination for low-risk individuals under 50 years old. Taiwan suspended 2nd Pfizer vaccine for ages 12-17 (please see below for detailed references to all risks of morbidity and mortality among those receiving COVID-19 mRNA vaccination compiled from Life Insurance Industry reports, VAERS database, U.S. Disability statistics and peer reviewed publications.)
A recent study in The Journal of Medical Ethics, an affiliate of the British Medical Journal, shows that getting a COVID-19 “booster shot” is at least 18 times more dangerous for young people than getting COVID-19. According to the study’s authors, “[t]o prevent one COVID-19 hospitalisation over a six-month period, we estimate that 31,207–42,836 young adults aged 18–29 years must receive a third mRNA vaccine. Booster mandates in young adults are expected to cause a net harm: per COVID-19 hospitalisation prevented, we anticipate at least 18.5 serious adverse events from mRNA vaccines, including 1.5-4.6 booster-associated myopericarditis cases in males (typically requiring hospitalisation).”
It is important to understand why these adverse events were only picked up in post-surveillance:
First, it must be recognized that the pediatric clinical trials for the COVID-19 vaccines were too small (the booster trial for 5-to-11-year olds had 140 participants) to detect safety signals for serious adverse events–especially for a recipient population in the tens of millions. It is difficult to understand how FDA allowed trials to be conducted with so few children enrolled, knowing they were inadequate to assure safety. Further, in the Pfizer trial, they vaccinated all the children in the placebo group after only a 6 week period, thus removing any ability to assess long-term safety.
Secondly, the Pfizer data presented to the FDA in support of an Emergency Use Authorization for vaccinating children from 6 months to 4 years old includes deeply concerning data regarding safety and efficacy. In a review by the diagnostic pathologist Dr Clair Craig, Co-Chair of the HART group (HART is a group of highly qualified UK doctors, scientists, economists, psychologists and other academic experts that came together over shared concerns about policy and guidance recommendations relating to the COVID-19 pandemic), she reports that:
- The trial recruited 4,526 children from 6 months to 4 years old. 3,000 did not make it to the end of the trial. This is a highly disturbing finding and is almost unprecedented to have this number of subjects (2/3) drop out of any trial. This level of drop-out essentially negates the value of any findings.
- They defined “severe COVID-19” as an increased heart or respiratory rate. There were 6 cases in the vaccinated group and only one in the unvaccinated group. The only child hospitalized in the trial had a fever and a seizure. They were in the vaccinated group.
- In the 3-week period between the first and 2nd doses in this trial, 34 of the vaccinated children contracted COVID-19, while only 13 in the unvaccinated group contracted COVID-19.
- In the 8-week gap between the 2nd and 3rd dose, again more subjects in the vaccinated group fell ill with COVID-19. These data were ignored.
- In the several weeks after the 3rd dose, again more subjects in the vaccinated group fell ill with COVID-19. These data were ignored.
- In the end they compared 3 children in the vaccine group who ultimately got COVID-19 post the 3-dose regimen and compared that number with the 7 children in the unvaccinated group.
- This was the basis for their claim of efficacy; however, it must be noted they ignored 97% of the cases of COVID-19 prior to this point.
- Further, of the 11 children who contracted COVID-19 twice during the trial, only one was unvaccinated. Many of the vaccinated children who contracted COVID-19 twice had received three doses already.
Furthermore, multiple case reports have suggested that vaccinating after infection increases the risk of vaccine-induced side effects such as myocarditis. Most concerning is this nationwide survey of myocarditis incidence in Finland from 2017 found that there were 4 cases per million.
The risks demonstrably outweigh the benefits of COVID-19 vaccination in children. A study out of Hong Kong showed one out of every 2,700 12-17-year-old boys are diagnosed with myocarditis following the second dose of Comirnaty vaccine (37 per 100,000 vaccinated). A study from Kaiser found the same rate of myocarditis in 12-17-year-old American boys, 1/2700. While CDC is saying that myocarditis is a mild disease, cardiologists know otherwise. The CDC’s own preliminary data reported at the February 4 ACIP meeting, revealed that nearly half of the young people diagnosed with myocarditis still had symptoms 3 months later, and 39% had their activity restricted by their physician. We know this serious adverse event frequently occurs in teenagers. But no one knows how often it occurs in younger children. This is of significant concern for babies and younger children.
There is no available care for children injured by COVID-19 shots. There is no way to remove the spike protein and other toxic byproducts of vaccination, which may be produced for a considerable period of time following inoculation of messenger RNA. The science and medicine have not yet developed, and most families will be unable to cover the costs of potential catastrophic injuries. The federal government’s Countermeasures Injury Compensation Program has not compensated a single person injured by COVID-19 vaccines.
Several months ago, FDA authorized booster doses of Pfizer vaccine for 5-11-year-olds without convening a VRBPAC meeting or providing any public discussion of the evidence supporting the booster. Dr. Peter Marks, the Director of FDA’s Center for Biologics, told the VRBPAC in April that the FDA’s issuance of an EUA for a second booster in adults was a “stopgap measure” – the implication being there was no scientific evidence to support that booster. Has FDA given up even the appearance of a scientific evaluation before issuing more EUAs for COVID-19 vaccines?
In the documents related to a recent FOIA request, in the Pfizer informed consent document it was revealed that the company recognized the risk of myocarditis to be as high as 1 in 10,000. In 2022, with many fewer vaccines administered compared to 2021, the rate of myocarditis reports to VAERS is averaging 245% higher than last year. The myocarditis is overwhelmingly found in children.
In a paper by Walach et al., the authors calculated the Number Needed to Vaccinate (NNTV) to prevent one death from a large Israeli field study. They then accessed the Adverse Drug Reactions database of the Dutch National Register (Lareb) to extract the number of cases reporting severe side effects and the number of cases reporting fatal side effects.
- They found the NNTV to be between 200 and 700 to prevent one case of COVID-19 by Pfizer’s mRNA vaccine product.
- The NNTV to prevent one death was between 9,000 and 100,000 (95% confidence interval), with 16,000 as a point estimate (as you will see below, for younger healthy people, this estimate would tend to the higher end of a NNTV of 90,000-100,000 to prevent a single death).
- They calculated that for every 6 deaths prevented by vaccination, there were approximately 4 deaths reported associated with vaccination, yielding a potential risk/benefit ratio of 2:3 (note that deaths are consistently under-reported to such databases, thus a more accurate risk/benefit ratio for death would likely be inverted).
- They concluded that, “although causality between individual reports of adverse events and vaccination has not been established, these data indicate a lack of clear benefit, which should cause governments to rethink their vaccination policy.”
In a published paper by Jessica Rose, a world expert analyst of the VAERS database, she found that, based on the ratio of expected severe adverse events to observed adverse events in VAERS for a number of conditions, the “underreporting factor (URF)” for COVID-19 vaccine-associated deaths was 31. Using this URF for all VAERS-classified severe adverse events, as of October 2021, vaccines were associated with 205,809 deaths, 818,462 hospitalizations, 1,830,891 ER visits, 230,113 life-threatening events, 212,691 disabled and 7,998 birth defects.”
A paper by Ronald Kostoff et al. was retracted despite passing peer review. However, in a personal review of the correspondence between the author and Journal Editor, neither I nor my colleagues were able to find a valid criticism of the underlying data analysis or conclusions. Therefore, I have incorporated this valuable study whereby they used a novel, best-case scenario, cost-benefit analysis which showed conservatively that there were five times the number of deaths attributable to each inoculation vs. those attributable to COVID-19 in the most vulnerable 65+ demographic. The risk of death from COVID-19 decreased drastically as age decreases, and the longer-term effects of the inoculations on lower age groups “may increase” their risk-benefit ratio (although this has not been demonstrated to date as can be seen below).
Current data shows that children have a 99.995% recovery rate, and a body of medical literature indicates that near nil healthy children under five years old have died from COVID-19. Further, only a fraction of the rare child deaths were due to COVID-19 and these do not accord with pediatric COVID-19 death rates from other countries. The NY Times has reported that the CDC has chosen to conceal the number of Americans who died due to COVID-19, even though the data are found on death certificates.
A study from Johns Hopkins that monitored 48,000 children diagnosed with COVID-19 showed a zero-mortality rate in children under 18 without comorbidities. The Wall Street Journal reported on this in their editorial comment “The Flimsy Evidence Behind the CDC’s Push to Vaccinate Children.”
A study in Nature demonstrated that children under 18 with no comorbidities have virtually no risk of death.
Data from England and Wales, published by the UK Office of National Statistics on January 17, 2022, revealed that throughout 2020 and 2021, only one (1) child under the age of 5, without comorbidities, had died from COVID-19 in the two countries, whose total population is 60 million.
A large study conducted in Germany showed zero deaths for children ages 5-11 and a case fatality rate of three per million in all children without comorbidities. A study published in December in Nature demonstrated how children efficiently mount effective, robust, and sustained immune responses.
The CDC published data stating that not one death occurred in children aged 6 months through 4 years old that was associated with COVID-19 during the late Delta through early Omicron wave from December 2021 through March 2022.
It is well known that hospitalizations and deaths with COVID-19 have been misattributed as hospitalizations and deaths due to COVID-19 by federal health agencies, leading to numbers of severe cases and deaths that have been disputed by US physicians investigating them, and which do not accord with the mortality rates for children in other nations. CDC now publishes its COVID-19 mortality data as deaths with COVID-19, blatantly exaggerating COVID-19-caused morbidity and mortality.
According to CDC and the New York Times, sampling data over a three-month term beginning in February 28, 2022 during which there has been fewer than one US child per 100,000 children hospitalized daily for COVID-19. Contrast this number with the 37 children per 1000,00 who will contract myocarditis from the vaccine as referenced above.
According to the CDC data tracker, less than 0.1% of all US deaths that have occurred “with” COVID-19 have occurred in children aged 0 through 4.
Strong evidence that newer variants of COVID-19 (Omicron) pose dramatically reduced risks to young children was published in the April 1, 2022, JAMA Pediatrics by Wang et al. Using a huge US medical database, they were able to match children aged under 5 who were infected with an Omicron variant with those who were infected with a Delta variant. Children with Omicron were only 35% as likely to require an ICU admission and only 15% as likely to require mechanical ventilation as same-aged children who had been sick due to earlier delta variants.
Below are the June 8, 2022, New York Times graphs for the current number of US patients in hospitals, ICUs, and suffering deaths attributed to COVID-19. The numbers of patients in ICUs dying each day ascribed to COVID-19 are close to the lowest numbers since the start of the pandemic. Given that CDC extrapolated that 95% of Americans already have partial to complete immunity, while we are at historic low levels for severe COVID-19 disease, it should be clear that there is no need to vaccinate anyone now.
The Pfizer clinical trials for children 2 through 4 years old failed to meet FDA-specified requirements for COVID-19 vaccine EUAs. The vaccines did not show 50% efficacy nor meet the required 30% lower bound with a 95% confidence interval Given these data, there is no support for the proposal to use a product and schedule that failed FDA’s established criteria in its clinical trials should not be allowed.
II. Real World Efficacy Data Raises Serious Concerns about the Benefit of mRNA Vaccines and Demonstrates that Vaccinated Individuals Are Likely at Higher Risk.
A. Numerous Studies Demonstrate Negative Efficacy.
Delving deeper into specific concerns about efficacy and further addressing the statements that “vaccination status, when you combine the benefits and the risks of it, it actually favors the unvaccinated” and that “all-cause mortality” is higher for vaccinated people than for non-vaccinated people, there are a number of proposed mechanisms for negative efficacy in the vaccinated. Dr. Paul Offit, Chair of the FDA Vaccine Advisory Board, for example conceded in a letter to the New England Journal of Medicine that there is a real concern of the shots inducing a form of immune suppression known as original antigenic sin.
Some of the evidence for negative efficacy comes from booster data, which have fleeting efficacy, as one data point the fact that the Pfizer shots in the 5-11 year range led to very poor efficacy; 31% according to the CDC and 12% after 7 weeks according to a massive database comprising over 1.3 million children (365,000 of whom were vaccinated) from the NY Department of Health. Five to 11-year-old children dropped into the negative efficacy range by 8 weeks after receiving the second dose. See Figure below.
This is the largest COVID-19 vaccine efficacy study in children ever published, using the highest quality, official data from NY state. There was a large, linear drop in efficacy seen with each successive week following full vaccination. Extremely narrow confidence intervals confirm the validity of these data. By 8 weeks following their second dose, vaccinated children were placed at higher risk of developing COVID-19 than unvaccinated children. By 9 weeks, their risk was even higher. Despite data-free theories offered to minimize this finding, the indisputable fact is that being vaccinated placed these children in a higher risk category for a COVID-19 infection than if they had never been vaccinated.
The original Moderna clinical trial data, which should have been available to regulatory agencies at least since the Moderna package was presented for licensure, reveals that while 93% of unvaccinated controls produced detectable SARS-CoV-2 anti-nucleocapsid antibody after infection, only 40% of the vaccinated produced this antibody after infection. Most of the vaccinated failed to mount the expected immune response. This is probably why Dr. Marco Cavaleri of the European Medicines Agency warned that frequent COVID-19 booster shots could adversely affect the immune response and may not be feasible. “Repeat booster doses every four months could eventually weaken the immune response and tire out people,” Bloomberg reported, quoting the European Medicines Agency. It is probable that the more doses of these vaccines you receive, the less broad immunity you will develop, even after getting infected.
Walgreens pharmacies perform rapid antigen COVID-19 tests and report weekly on the results, based on the number of vaccine doses received and the date the most recent vaccination was obtained. In June 2022, their data revealed that receiving a 2nd or 3d dose within the past 5 months leads to a comparable positivity rate as being unvaccinated (21.8-26.2%). However, receiving 2 or 3 doses more than 5 months ago leads to the highest positivity rates (33.5-38.4%). This is further supportive evidence that efficacy falls into negative territory several months after vaccination. See the chart below.
With the above caveats in mind, the data indicates that vaccinated individuals are more likely to fall ill with the variants now in circulation. This may not have been the case earlier in the global vaccination campaign but is unfortunately the case now. There are several possible explanations for this finding. Chief among them is that the current mRNA vaccines were formulated using the genetic sequences of the original “Wuhan” strain of SARS-CoV2 from over 2 years ago. Given SARS-CoV2 is a highly mutagenic virus, many dozens of variants have since emerged, with several strains exhibiting sudden, multiple, and major pathogenically important mutations, particularly within the original spike protein to which the mRNA sequences are directed.
The major mutations have been “named” and each have many subvariants. The Delta variant phase in the U.S. ran from approximately June of 2021 to January 2022, after which the Omicron variant has predominated, and we are currently seeing rising cases from sub-variants of this strain. Omicron deserves mention as it is phylogenetically different from both Delta and the original Wuhan strain. This is likely the most accurate explanation as to why, in the setting of what are now “non-neutralizing” antibodies, this paradoxically makes “Wuhan strain” vaccinated individuals more susceptible as follows;
Stanford researchers found that “prior vaccination with Wuhan-Hu-1-like antigens followed by infection with Alpha or Delta variants gives rise to plasma antibody responses with apparent Wuhan-Hu-1-specific imprinting manifesting as relatively decreased responses to the variant virus epitopes compared with unvaccinated patients infected with those variant viruses.”
From a Public Health England vaccine surveillance report in the U.K., government researchers asserted that their serology tests were underestimating the number of people with prior infection due to recent observations from UK Health Security Agency (UKHSA) surveillance data that “N antibody levels appear to be lower in individuals who acquire infection following 2 doses of vaccination.”
In this peer-reviewed paper they found that at the country-level (and U.S county level), there appears to be no discernable relationship between the percentage of the population fully vaccinated and new COVID-19 cases as seen below. In fact, the rising slope of the relationship in both graphs below suggest that mass vaccination policies may paradoxically lead to more cases, with Israel serving as a worrying outlier.
This is also seen in a European Journal of Epidemiology study that found that at the country-level, there appears to be no discernable relationship between percentage of population fully vaccinated and new COVID-19 cases in the last 7 days (Fig. 1). In fact, the trend line suggests a marginally positive association such that countries with higher percentage of population fully vaccinated have higher COVID-19 cases per 1 million people.”
A study prepared by Humetrix for the Department of Defense called “Project Salus,” monitored 20 million Medicare beneficiaries from January to August of 2021 and found that the vaccinated share of the COVID-19 hospitalizations rose steadily with both vaccines after three to four months and sharply after six months (as the Israelis found). By late July, 71% of all cases and 61% of all hospitalizations were among vaccinated individuals.
More current data from the Walgreens chain of pharmacies finds that in the U.S, over the last several months, fully or partially vaccinated individuals are testing positive at higher relative rates than the unvaccinated.
According to Cornell University’s faculty, an outbreak in December of 2021 that forced the school to switch to online learning was driven exclusively by the vaccinated. “Virtually every case of the Omicron variant to date has been found in fully vaccinated students, a portion of whom had also received a booster shot,” said Vice President for University Relations Joel Malina in a statement.
On December 31, 2021, the UK’s Office of National Statistics released an “Infection Survey” of 1,701 individuals who tested positive for COVID-19 between Nov. 29 and Dec. 12, of whom 115 tested positive for the Omicron variant. The agency found a clear correlation between the number of vaccinations and the likelihood of an Omicron-positive result. The odds ratio of testing positive for Omicron with two vaccinations was 2.26; for the triple-vaccinated, it was 4.45.
According to the latest U.K. health surveillance report, roughly 95% of those over 70 are double-vaccinated and about 90%-93% of the age cohorts over 70 are boosted. Just 1.6% of the senior cases between weeks 7 and 10 of this year were among the unvaccinated, which is below the 5% share of the population they compose. The triple-boosted actually made up 90% of the cases.
The respected Robert Koch Institute reported that among the 4,206 Germans infected with Omicron for whom their vaccination status was known, 95.58% were fully vaccinated. More than a quarter of them had booster shots. Given that the overall background rate for vaccination in Germany is 70%, this suggests an -87% effectiveness rate against Omicron.
As of Dec. 31, 2021, in Denmark, 89.7% of all Omicron cases were among the fully vaccinated with just 8.5% of all cases in Denmark among the unvaccinated, according to the Statens Serum Institut. Overall, 77.9% of Denmark was fully vaccinated at the time, and Omicron is more prevalent among younger people for whom there is a greater unvaccinated pool, which again support a negative efficacy. Even for non-Omicron variants, the unvaccinated composed
only 23.7% of the cases.
______________________________________________________________________
B. The Evidence Alleging to Show Significant Reduction in Severity and Mortality from the mRNA Vaccine Is Overstated.
There have been a numerous reports that large numbers of COVID-19 deaths are preventable using mRNA vaccines, an element in the support for the use of coercion against medical professionals via licensing and credentialing actions to ensure compliance. An example is a 2021 analysis that utilized a number of questionable assumptions to conclude that higher vaccination rates could have saved over 200,000 lives. The impact of mRNA vaccination on longevity is discussed throughout this section, but it is useful to highlight some of the faults in such studies:
- Since so many deaths not caused by COVID-19 have been classified as COVID-19 deaths, we don’t actually know how many people died from the illness, including deaths that could have been caused by mRNA vaccination (this study just assumed the official but inflated figure as accurate).This is a complex topic about which there are professional differences of opinion and the evidence is not yet fully clear, in part because of difficulties obtaining data from CDC and FDA as they have opposed release of information in court and were concerned that the data would be “misinterpreted” as showing unacceptable risks of vaccination.
- The study ignores numerous confounding variables that could account for different death rates such as differences in implementation and compliance with other public health measures, along with other limitations noted in the study.
- In Pfizer’s trial, the survival benefit from the vaccine worsened with time (this has also been observed outside the trials), and at 6 months follow-up (where the trial was abruptly terminated), more people who were vaccinated died than those who were unvaccinated (which means that it is impossible that there could have been a net gain of life through vaccinating). Since this is the longest clinical trial that was performed on the vaccines, its conclusion must stand until a longer trial is conducted.
- The vaccines we are using have caused SARS-CoV-2 to rapidly evolve into variants for which it no longer offers protection. For this reason, the alleged benefits of the vaccine have had to be continually modified because the vaccine failed to meet each of its previously promised metrics (i.e., it does not prevent transmission of COVID-19).
- The study fails to account for the fact that national death rates consistently increased or stayed the same (but never decrease) following COVID vaccination campaigns:
- The estimate fails to account for studies like the recent one from the Cleveland clinic, which have found that the risk of contracting COVID-19 increases with the number of vaccines received:
- The estimate also fails to account for the fact that life insurance data have shown an unprecedented spike in deaths following the mass vaccination campaigns for age groups rarely expected to otherwise die, (data summarized here).
- Because any immunity derived from mRNA vaccination is so short-lived, and unlike original expectations continual boosters are required, the risks presented by vaccination are increased.
This short discussion does not do this topic justice, the interpretation of vaccine effectiveness data is highly complex and by no means is the full story known. Much of the risk/benefit discussion, for example, conflates relative with absolute risk which skews the apparent benefit at the same time that much of the literature underplay the risks.
C. The Evidence Also Demonstrates that Vaccination is Not Effective in Preventing Severe Disease
The efficacy of the COVID-19 mRNA vaccine in preventing severe disease is also questionable. CDC data shows that there is no statistically valid evidence that they prevent severe disease or deaths in children. Current mRNA injections were formulated based on the original Wuhan strain and were not tested for benefits against current variants in clinical trials. Which raises the question as to what can be accomplished by vaccinating small children with an outdated vaccine.
In Ireland, in March of 2022, during the milder Omicron variant wave, there were more people in Irish hospitals than at any point in the previous 12 months. This occurred despite the fact that nearly 95% of all adults in Ireland are fully vaccinated, and nearly 100% of seniors are vaccinated and boosted.
In Israel, the Director of a major hospital recently declared that the fully vaccinated are not protected against severe illness.
NSW Health in New South Wales, the most populated of Australian states at 8.1 million inhabitants, reported that 97 out of 98 COVID-19 deaths occurring over the previous two weeks involved fully vaccinated persons. Moreover, those that had three doses appeared most at risk for hospitalization admission, ICU transfer, and death.
These data are consistent with the recent report published in the New York Times, which stated, “despite strong levels of vaccination among older people, COVID-19 killed them at vastly higher rates during this winter’s Omicron wave than did last year, preying on long delays since their last shots and the variant’s ability to skirt immune defenses.”
The conclusion of a recent Danish study in the prestigious Lancet a pre-print study found that in long-term follow-up of over 74,000 adult participants in the Moderna and Pfizer trials there was no all-cause mortality benefit from the two mRNA shots.
In a recent, large Veterans Administration study, investigators discovered disturbing evidence: by month six after a SARS-CoV-2 infection, beyond the first 30 days of illness, vaccinated persons with breakthrough infections were at higher risk of death (hazard ratio (HR) = 1.75, 95% confidence interval: 1.59,1.93).
The implications of the vaccine’s diminished ability to protect against severe disease among more recent variants is now playing out in real-time. On June 5th, 2022, analyst Igor Chudov posted a 2 country comparison of the current cases and deaths being reported from Portugal and S. Africa, two countries undergoing similar waves of infection from the emerging B4/5 sister variants. South Africa is only 35% vaccinated and 5% boosted whereas Portugal is 95% vaccinated and 70% boosted. These variants are now driving a deadly wave of COVID-19 in highly vaccinated Portugal, with deaths among the Portuguese nearing their January peak and still rising as seen below.
Thus, in terms of benefits, based on the most up-to-date data, the current crop of mRNA vaccines against Omicron confer either rapidly waning efficacy or negative efficacy, and not only do they no longer protect against severe disease, but it appears to be raising the risk of severe disease and death.
D. Vaccination Also Does Not Appear to Prevent Long-Haul COVID-19.
Finally, the efficacy of the COVID-19 mRNA vaccine in the prevention of “long-haul” COVID-19 does not appear to be supported. A large Veterans Administration study recently reported disturbing evidence: by month six after a SARS-CoV-2 infection, vaccinated persons with breakthrough infections were at higher risk of long COVID-19 (HR = 1.50, 95% CI: 1.46, 1.54). When including the earlier time periods, the COVID-19 vaccines only reduced the risk of long COVID-19 by approximately 15% compared to the unvaccinated, a level of estimated protection far less than the increased risk of death found in the same study as mentioned above.
Again, from the large Veterans Administration study, investigators discovered disturbing evidence: by month six after a SARS-CoV-2 infection, vaccinated persons with breakthrough infections were at higher risk of long COVID-19 (HR = 1.50, 95% CI: 1.46, 1.54). When including the earlier time periods, the COVID-19 vaccines only reduced the risk of long COVID-19 by approximately 15% compared to the unvaccinated, a level of estimated protection far less than the increased risk of death found in the same study as mentioned above.
III. Natural Immunity Appears to Be Superior than mRNA Vaccination And, in Light of Potential Risks, Supports the Reasonable Position That the Vaccine Should Be Deferred in Patients with a Positive History.
Natural immunity provides robust protection, not only from contracting the COVID-19 a second time, but also against hospitalization and death. Given the adverse events noted in this Section, this calls the policy of those with a prior COVID-19 history into serious question.
Beginning with the concern for the young, most children are already immune. Natural immunity is superior to vaccine-induced immunity and vaccinating the already immune is superfluous and potentially harmful as per this study. Further, CNBC reported in March 2022, “an estimated 95% of Americans ages 16 and older have developed identifiable COVID-19 antibodies.” CDC earlier said over 75% of children already have partial or full immunity to COVID-19.
The most recent review of data supporting the protection of natural immunity, compiled from over 160 research studies, found that natural immunity provided equal or superior protection against not only contracting the disease, but also against hospitalization and death.
Further, vaccinated individuals are far more likely to get re-infected with COVID-19 compared to those with natural immunity. A new preprint study from Bangladesh found that among 404 people re-infected with COVID-19, having been vaccinated made someone 2.45 times more likely to get re-infected with a mild infection, 16.1 times more likely to get a moderate infection, and 3.9 times more likely to be re-infected severely, relative to someone with prior infection who was not vaccinated. Although overall re-infections were rare, vaccination was a greater risk factor of re-infection than co-morbidities.
A new study from Harvard, Continued Effectiveness of COVID-19 Vaccination among Urban Healthcare Workers during Delta Variant Predominance tracked vaccinated and unvaccinated Massachusetts healthcare workers and showed 0 infections in 74,557 person-days for previously infected patients compared to 49 infections out of 830,084 person-days for fully vaccinated patients.


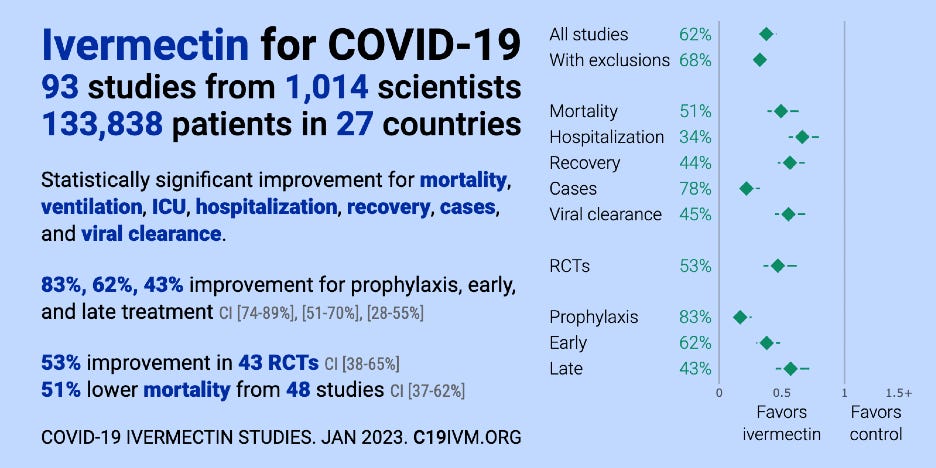



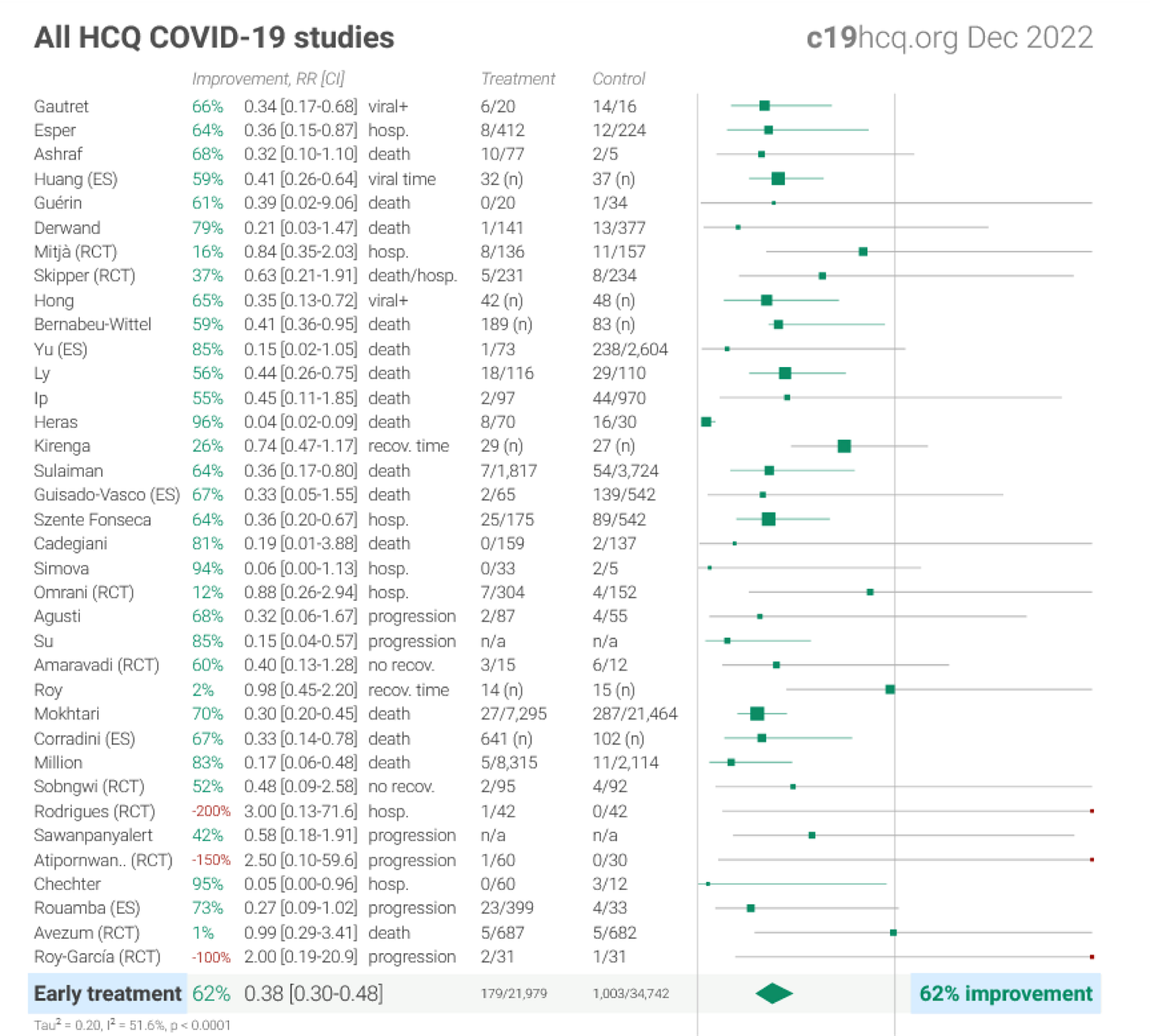




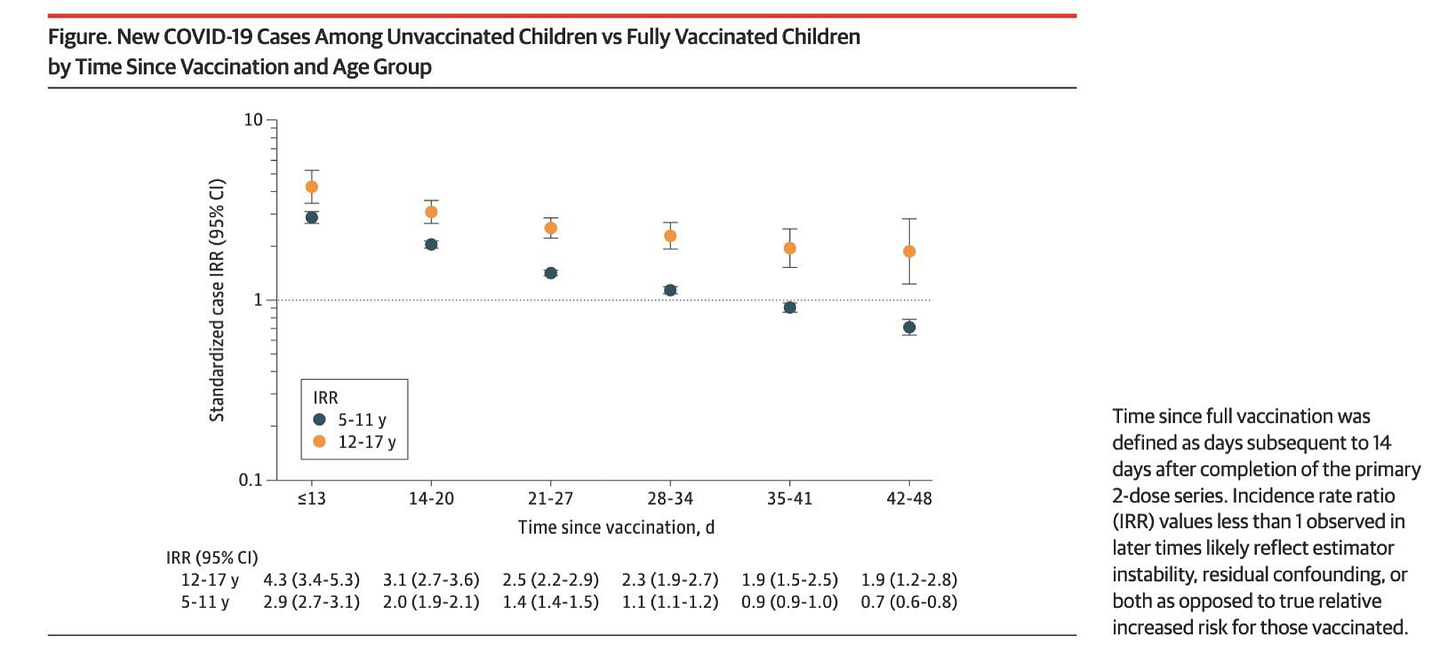


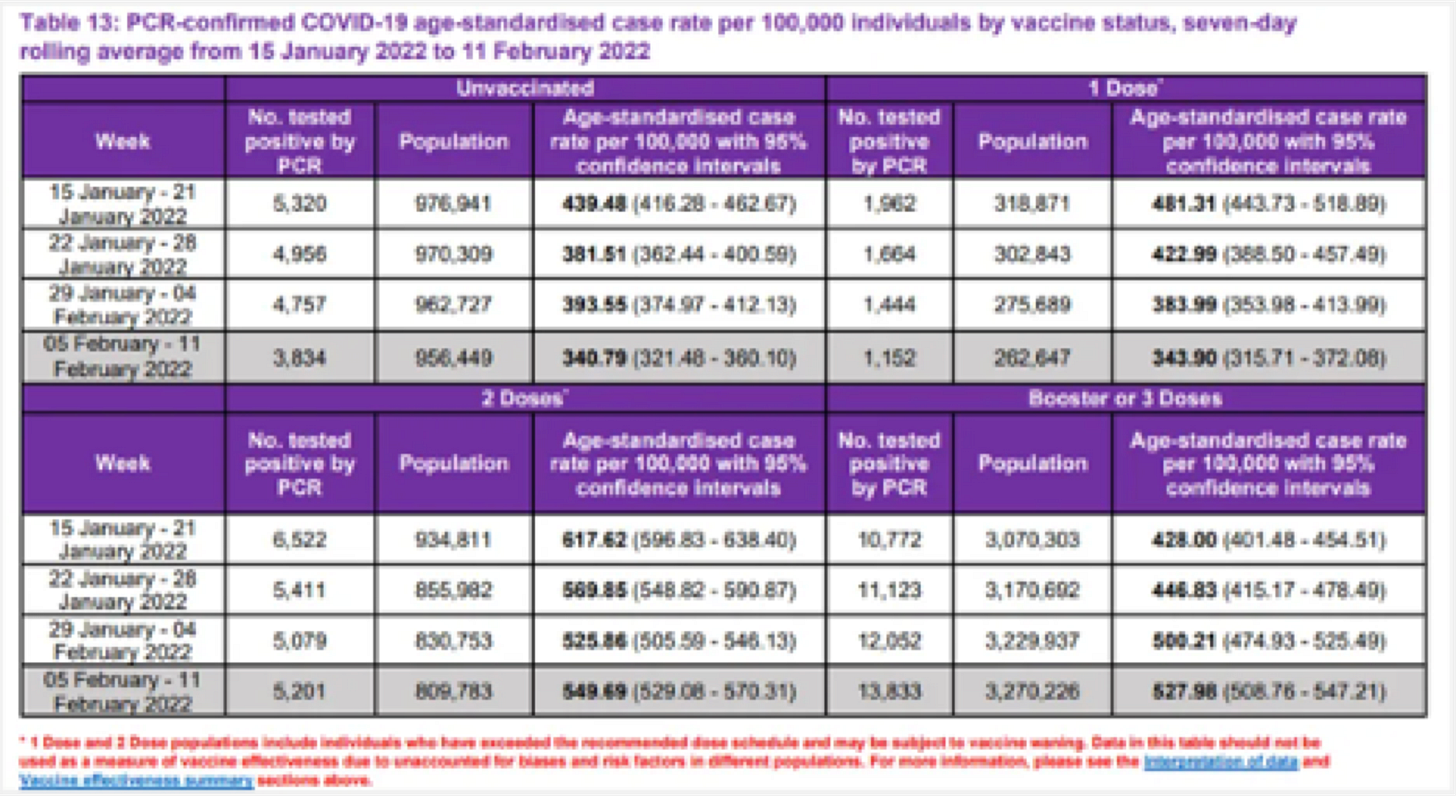
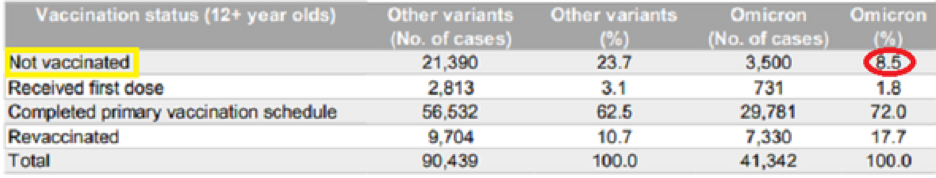

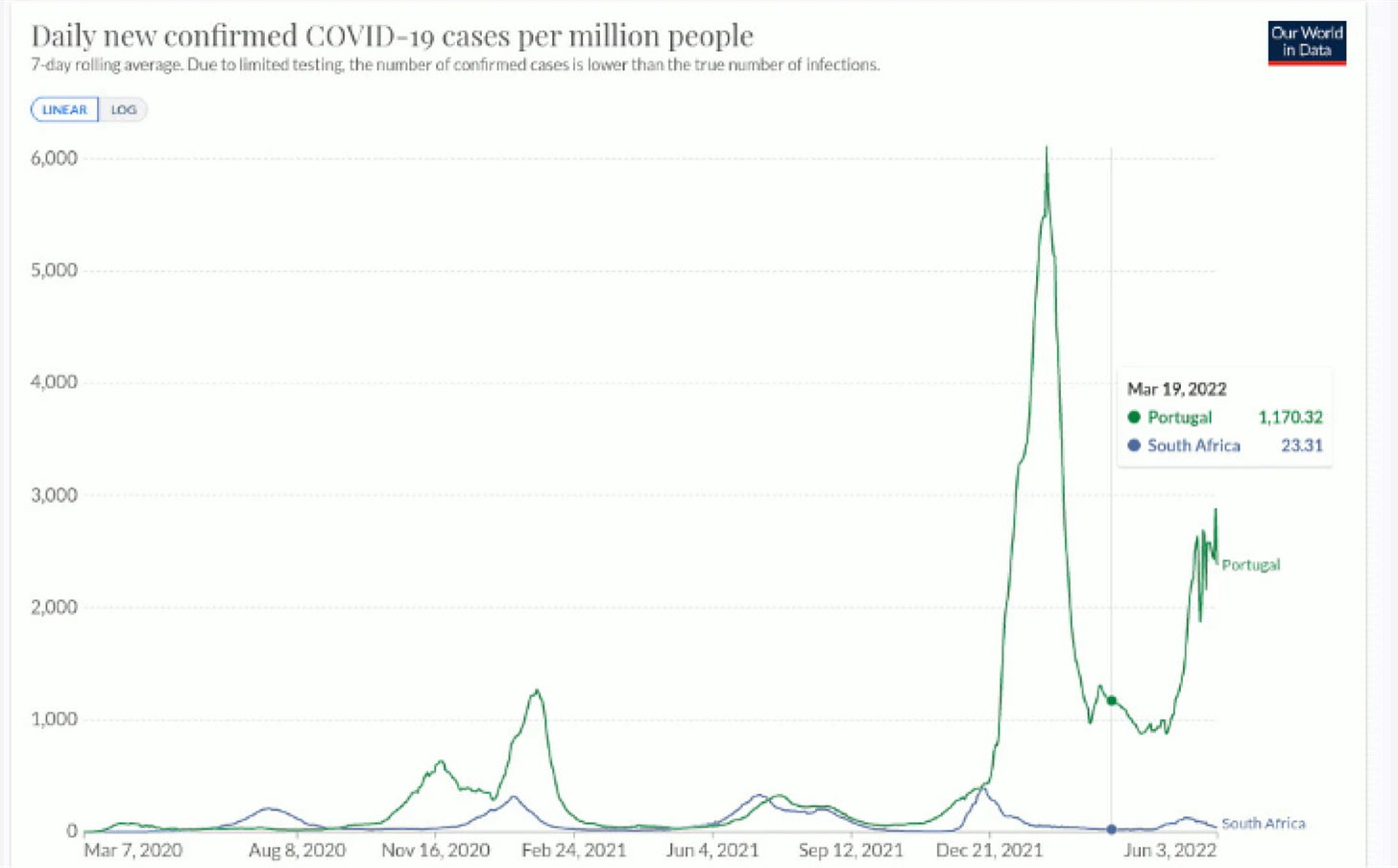
Shouldn't we all write in to the board? Dr. Kory and Dr. Marik are representing all of us. They are true American heroes. We all stand to lose if they lose. I suggest we aim to publicize this and make our voices heard. I'm sure many who would not become involved in the intricasies of the case would support their rights in this case.
This was political, never scientific. They have shown us who they are. We have your back. Medicine will never have the same esteem in the eyes of millions of members of the public.