Chapter 16 - The Six Faces of Earth’s Minerals: Not All Are Created Equal
Brine, ocean, seaweed, soil, and stone—how the origin of a mineral supplement determines its power, purity, and purpose.
Here you will learn more about the various sources of trace minerals than you ever wanted to, and some of it may make you uncomfortable.
Here you will learn more about the various sources of trace minerals than you ever wanted to, and some of it may make you uncomfortable.
Ocean Water Minerals
“Quinton minerals” are sourced from defined sites of deep ocean water, (often during plankton blooms); microfiltered rather than chemically extracted. They contain up to 78–80 minerals and trace elements in their natural ionic form, closely mirroring the mineral balance and ratios found in human plasma.
The water primarily includes chloride, sodium, magnesium, potassium, calcium, sulfate, and trace levels of other elements, with the minerals present as natural marine salts with no phytonutrients as you get from plant mineral sources. Not all minerals occur in a sulfated form as there are various other anions (chloride, sulfate, bicarbonate, etc.) that naturally occur in seawater.
Seawater itself contains only trace amounts of metals like iron and aluminum, since these elements are largely insoluble under open-ocean conditions. In contrast, marine fulvic substances and ocean-floor mineral sediments are rich in these and other transition metals.
It is within these boundary layers — where organic matter, minerals, and redox-active metals interact — that natural purification and catalytic reactions occur, processes that seawater alone cannot sustain in solution. These are the same mineral interfaces that Asao Shimanishi reproduced through his Themarox process — the engineered recreation of the ocean floor’s catalytic environment in a concentrated, ionic form.
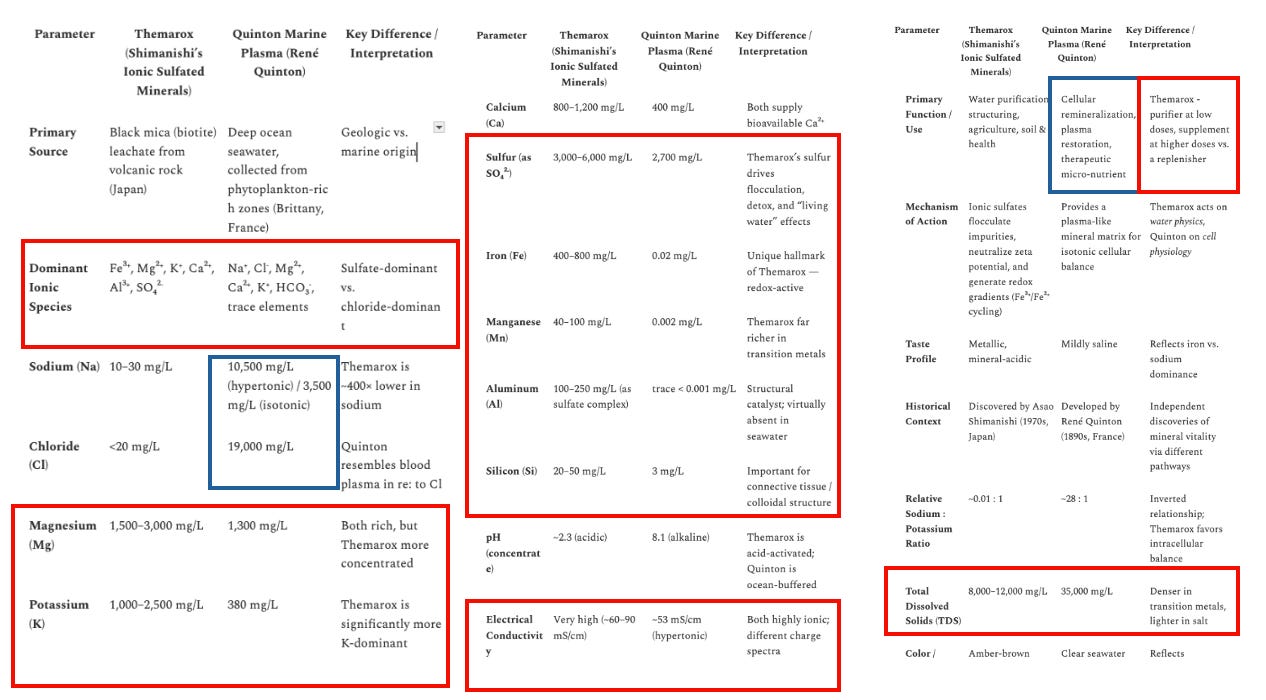
In the below table, red boxes indicate the parameter where I find Themarox superior, blue is where Quinton is superior.
Themarox Compared to Quinton Minerals
Red boxes indicate the parameter where I find Themarox superior, Blue is where Quinton is superior.
*Note the pH of Themarox, although accurate in its original concentrated state, is not relevant to Aurmina in water, where it is only slightly acidic and keeps pH balanced.
Great Salt Lake Mineral Concentrates
The “Veggie Trace Minerals” that Elmer Heinrich praised in his book “The Untold Truth” are from brine concentrated from a geologic salt lake (not from plants as he described them), although they are sometimes marketed as “plant-based” because the trace spectrum resembles what plants use.
These supplements are produced by concentrating and purifying natural brine from the Great Salt Lake. The minerals are present primarily as inorganic ions and salts (e.g., magnesium, sodium, potassium, sulfate, chloride), not as plant-derived chelates. Because they’re already in ionic form, they dissolve readily and can be dosed in small amounts.
These products — like other “top” trace mineral supplement products you can find online — are generally derived from the Great Salt Lake basin. Usual concerns are heavy metals/halides (e.g., As, Pb, Hg, Br⁻) and purity—not provenance.
Main differences between lake derived and black mica are that in the former, iron is typically low and although sulfate is present, it is not dominant. Thus, the lake derived has no flocculation/coagulation effects - cannot be used as a water purifier. Lastly, in terms of rare-earth elements, they are usually in the amounts of trace to none.
Themarox vs. Great Salt Lake Minerals
In the below, red boxes indicate the parameter where I find Themarox superior, Blue is where Quinton is superior.
Plant/Seaweed–Derived Minerals
Plant/seaweed mineral products come from current biomass—harvested kelps and other seaweeds, calcified red algae (e.g., Lithothamnion), and nutrient-dense greens like spirulina/chlorella or moringa—then extracted. They do not originate from ancient decomposed remains. In these materials, minerals reside inside plant tissues, often bound to organic ligands (amino acids, polysaccharides) or, for red algae, locked into calcium–magnesium carbonates.
Seaweeds: strong in iodine, plus Mg, K, Ca.
Red algae (e.g., Lithothamnion): primarily Ca–Mg carbonate with 70+ trace elements.
Greens (spirulina/chlorella/moringa): background K/Mg/Ca with modest iron. Alongside minerals, these extracts carry phytonutrients—polyphenols and phlorotannins in seaweeds; pigments/antioxidants and fibers in greens—making them “food-form” supplements that are typically gentle for daily augmentation (targeted iodine or Ca/Mg, plus broad trace support).
Because composition varies by species, harvest site, and season, batch variability is real; rigorous metals/iodine controls are essential. Watch for inorganic arsenic and excess iodine in seaweeds, and Pb/Cd depending on waters; for greens, ensure screening for pesticides and microbial contaminants.
Another comparison table - seaweed hits some high points (very high iodine magnesium, potassium content) but some low ones (high calcium, high sodium, less sulfur less iron). Ultimately it is not as uniquely balanced as Themarox)
Themarox vs. Plant/Seaweed–Derived Minerals
Key limitation vs. black-mica minerals
Plant/seaweed extracts are lower in iron and low in sulfate, and—critically—do not exhibit the electrochemical water effects seen with black-mica–derived sulfated ionic minerals (e.g., Themarox). They don’t drive flocculation/coagulation, don’t markedly raise ORP, and won’t reshape water’s charge dynamics. In short: excellent for food-like mineral top-ups and phytonutrients, but not for sulfate/iron-centric chemistry or water-treatment behaviors.
When to use which
Choose plant/seaweed minerals for a nutritional top-up—iodine from kelp, Ca/Mg from red algae, or broad trace + antioxidants from greens. Choose black-mica (sulfated ionic) minerals when you specifically want iron and sulfate delivery and/or water-chemistry effects (charge balancing, ORP increase, flocculation) that plant/seaweed products simply don’t provide.
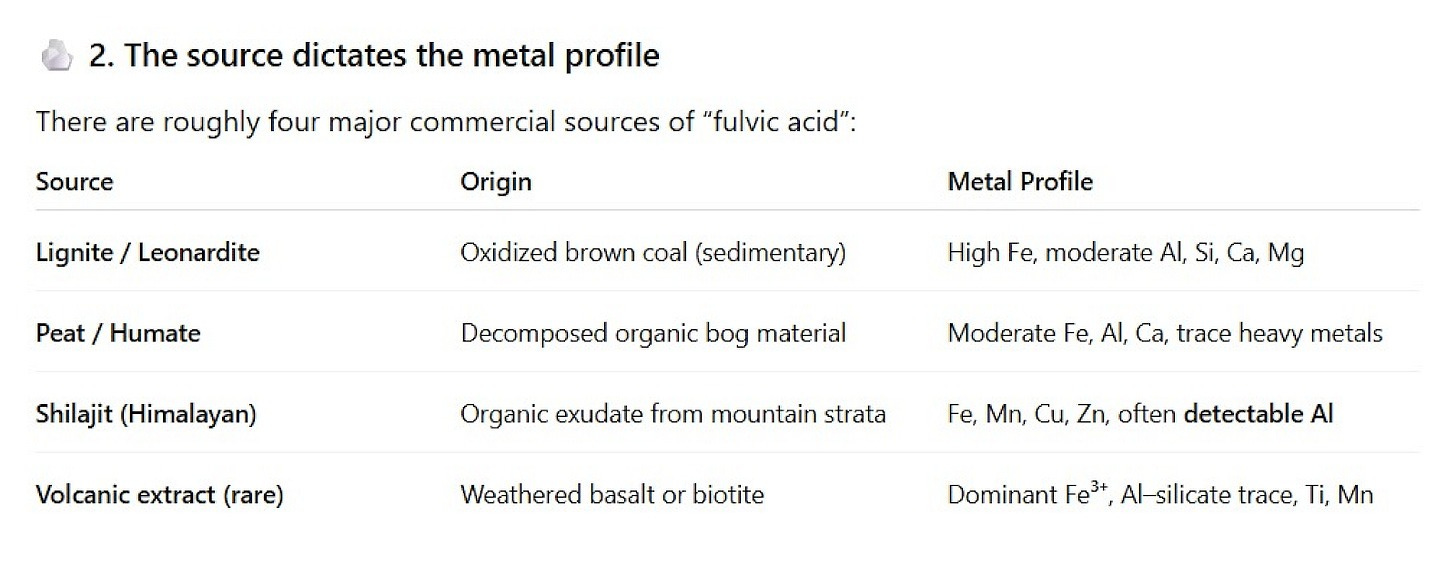
Fulvic & Humic Acid Minerals
Fulvic and humic acids are natural components of humus—the dark, complex material formed as plants, animals, and microbes decompose over long time scales. At least that is the common conception of them, but in reality, fulvic and humic acids don’t only originate from terrestrial soil humus — they’re also widely distributed in aquatic environments, including lakes, rivers, estuaries, and ocean sediments.
Commercial supplements extract these fractions (often from leonardite/humates) and concentrate the associated organic complexes bound to minerals. Unlike purified inorganic salts like Shimanshi’s minerals below, these products are organic–mineral mixtures whose composition reflects the source deposit and extraction method.
Sources and Compositions
The problem with the above is that when you see “Fulvic Trace Mineral Complex” on a label, that number usually refers to an optical density or extraction yield, not an elemental analysis. There’s no standardized molecular formula for fulvic acid — it’s a mixture of thousands of carbon-based fragments that chelate metals. Its real composition depends entirely on what the starting material was. Note the ubiquitous presence of aluminum (not because it is bad (see Chapter 17 for the “lowdown” on aluminum in foods); here, I am pointing it out to reveal how aluminum is literally the driver of its formation).
A respectful note on origin
Despite being widely marketed as ‘plant-derived,’ fulvic acid is in truth the product of microbial transformation of both plant and animal matter over geological time. Because humic deposits arise from indiscriminate natural accumulation of biological matter, the original inputs are not traceable to specific species. While modern processing transforms that material into chemically altered humic substances, it is scientifically accurate—and respectful—to acknowledge that ancient animal, and potentially human, remains may have contributed to the source of organic matter. The final product does not contain recognizable tissues, but its deep-time origin is biological, not geological.
Although some maintain that since leonardite/lignite-derived fulvic/humic products come from geologic deposits of “ancient” plant material formed millions of years ago, they do not contain human remains. Although such a claim might be logical on its surface, the fact is that Fulvic acids are variable organic complexes derived from decayed biological matter — their exact age and biological origin are indeterminate despite the fact that that the lignite or leonardite deposit it was mined from formed millions of years ago.
Further, for other biogenic sources (peat, soil, sediment, and shilajit), these are much younger accumulations of decomposed biological matter, so it is not possible to rule out contributions from animals—and, in principle, humans—at the origin site, even though the final extracts contain no identifiable tissues. With shilajit, the key is that the contributing biomass reflects whatever lived (and died) in that landscape. In high alpine zones, that is overwhelmingly plants and microbes, but if from lower or mixed remains, animal inputs can occur.
Strengths (when quality is verified)
Broad spectrum of trace constituents: Often contain a wide array of trace minerals and organic ligands inherent to humus chemistry.
Natural chelation & transport: Fulvic acid can bind minerals, potentially enhancing solubility and cellular transport (a reason for its popularity in agriculture and supplementation).
Binding capacity: Humic/fulvic matrices can bind various ions; some practitioners value this for supporting elimination of certain metals or metabolites (evidence base varies by endpoint).
Limitations & risks
Variability: Composition varies widely by deposit and process; two bottles from different sources may differ materially in mineral profile and organic residues.
Contaminants: Without rigorous sourcing and testing, products may contain excess metals (e.g., Pb, As, Cd) or unwanted organics.
Label ambiguity: “Fulvic content” is sometimes overstated or undefined; look for standardized assays (e.g., ISO/standardized fulvic quantification), full metals panels, and batch COAs.
Elemental spectrum: Compared to igneous/metamorphic, rock-derived mineral solutions, humic sources are less likely to contain certain rare-earth elements and may show different redox behavior.
Practical guidance for readers
Insist on documentation: Choose brands with GMP certification and third-party COAs for each lot (identity, microbial, full metals including As, Pb, Cd, Hg, and aluminum, plus organic contaminants where applicable). Look for proven geographic sources and altitude.
Check standardized measures: Look for defined fulvic/humic quantification methods rather than marketing percentages.
Start low, go slow: Given variability, begin with conservative dosing and monitor tolerance.
Match to intent: If your priority is a predictable mineral spectrum, a rock-derived ionic formulation may be preferable; if you’re seeking organic chelation properties, a validated humic/fulvic extract might suit, provided quality proofs are in place.
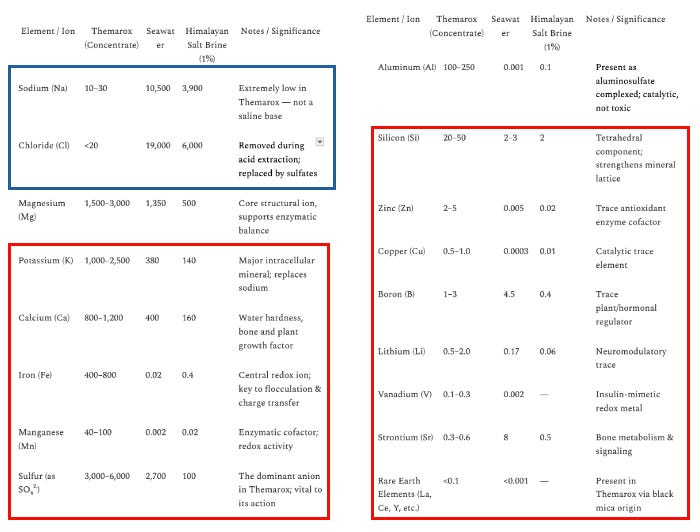
I leave you with yet another comparison table, note I found Themarox superior in the below parameters, and didn’t even mention that rare earth and ultratrace are likely superior as well.
Themarox compared to Fulvic/Humic
Sea Salts
This book was about to push off to the printer when I saw a question from a reader on my blog, “I like to supplement my trace minerals with Pink Himalayan Sea Salt.”
For those who depend on it or are wondering whether Pink Himalayan Hea Salt (PHSS) provides a broad range of trace minerals, I found that it does! Using AI, I found the mineral profile to be almost tantalizingly good (but that is where the good news ends). Check out what is in PHSS:
Major Minerals:
Sodium, chloride, calcium, magnesium, potassium, sulfur, iron, and phosphorus are the principal mineral constituents of Himalayan pink salt.
Trace Minerals:
Zinc, manganese, copper, iodine, fluoride, selenium, chromium, molybdenum, cobalt, nickel, vanadium, strontium, boron, and lithium occur in smaller but measurable amounts.
Rare Earth Elements:
Barium, rubidium, cesium, yttrium, lanthanum, and cerium are present at ultratrace levels, reflecting the salt’s deep geologic origin.
Problem: The amounts of the trace, ultra trace, and REE’s in the PHSS are negligible and in sea salts and thus would do zilch in terms of being able to supplement in any physiologically meaningful way. For instance, the mineral content of most other trace minerals is 1/100th to 1/1000th that in Themarox.
So, it could never be a supplement of diverse minerals because it is so high in sodium and chloride, you would be severely limited in your ability to ingest enough to elevate all the other minerals to “supplemental” levels.
In fact, the table below is a really, really good illustration of how jam-packed with diverse minerals Themarox is compared to PHSS (check out AI’s summary comparison at the end), and further, how little sodium chloride Themarox contains.
Pay particular attention to Themarox’s uniquely high amounts of iron, sulfur, magnesium, potassium, manganese, copper, boron, lithium, vanadium, and strontium - when you look at sea salt, it has nowhere near (and I mean nowhere) its potency as a supplement.
Now, if you think the aluminum content in Themarox is a problem, then I can immediately deduce two things about you:
1) You are no different than anyone else on Planet Earth that I have met in terms of misplaced fears around aluminum (and were like me before I wrote this book),
2) You haven’t read Chapter 17: Aluminum—From Feared Toxin to Forgotten Ally
The values below synthesize Shimanishi’s patent data, the 2012 SWAPE ICP-MS report, seawater standards, and common Himalayan salt analyses.
Summary of Key Differences
Sodium-Chloride vs. Sulfate Base
Themarox is a sulfate-dominant mineral complex, whereas seawater and Himalayan salt are chloride-dominant. This gives Themarox very different chemical behavior — it flocculates impurities, binds metals, and structures water rather than simply adding salinity.
Multivalent Ion Profile
High Fe³⁺, Mg²⁺, Ca²⁺, Al³⁺, and SO₄²⁻ content creates powerful electrostatic and catalytic properties — core to its water purification and biological resonance effects.
Low Sodium
At <0.003% Na, Themarox behaves more like a micronutrient solution than a salt. It’s safe for plant, animal, and human application without osmotic stress.
Rare Earth Element (REE) Trace Signature
Unique among mineral concentrates; stems from biotite’s deep-crustal origins — Y, La, Ce, Nd traces align with Shimanishi’s “life-code” hypothesis.
Functional Implication
Themarox = ionically charged, low-salt, high-reactivity medium.
Seawater/Himalayan salt = static electrolyte storage medium.
In addition to all the above limitations of PHSS, there is an even more concerning one:
Although Himalayan pink salt is generally safe, some samples have shown trace or excessive heavy-metal contamination—including lead levels above legal limits in one study and detectable arsenic, cadmium, and aluminum across brand in another—so purity testing and verified sourcing remain essential.
Black Mica Derived Minerals
Black mica-derived minerals, unlike organic plant powders, come in a liquid form of naturally occurring, water-soluble (sulfated) minerals in an ionic (charged), inorganic (no carbon) matrix. Thus, they are highly bioavailable and bioactive. Unlike other plant-based mineral complexes above, it also contains a broad spectrum of minerals, including some rare-earth elements. Further, they provide a richer, more unique mineral profile than soil-based sources—especially sulfur, iron, magnesium, potassium, and trace rare-earth elements. Importantly and uniquely, they are “sulfated,” and thus dissolvable in water. Further, the high sulfate content powers several of the many biochemical mechanisms detailed in Chapter 14.
Black Mica Mineral’s Unique Effects On Water
The black mica-derived mineral complex is of geological origin (rather than organic), and thus stands apart. Unique to this particular mineral complex, it contains both iron and aluminum sulfate which are known “flocculants” - i.e. they can bind contaminants in any water source and cause them to precipitate. Thus you then can easily filter out any harmful toxin that might be present in the water you add them to.
As we covered previously, since the minerals are all electrically charged, they can interact chemically with water molecules which influences the structure and chemistry of the water—something plant-based minerals cannot do—benefiting both the environment (through cleaner water) and, indirectly, the body, through improved mineral balance and the effects of structured water.
A widespread belief is that the best water to drink is good mineralized, spring water. Without minerals, water is “dead.” With ionic minerals, it gains structure, vitality, and becomes biologically supportive. If you look at true spring water, the minerals that give it life are mostly inorganic ions—calcium, magnesium, sulfates, silicates—not plant-based compounds. Even the ocean, which sustains the richest ecosystem on earth, is full of inorganic minerals. Without those ions, life in the sea could not exist.
And since pristine, mineral-rich springs are increasingly hard to access, remineralizing our water is no longer optional—it’s essential. Black mica minerals restore the ionic balance nature uses to sustain springs and oceans, turning ordinary or “dead” water into water that is living, structured, and safe.
The Future Of Themarox-Derived Minerals As A Potential Dietary Supplement For Humans
First, let me be absolutely clear: Themarox itself is a raw material, not a finished dietary supplement. Nothing derived from it is currently marketed, sold, or intended for human supplementation.
The water purification products made from Themarox —such as Aurmina (the company I started in the wake of this book), or its predecessor, AdyaClarity —are made by diluting Themarox with 9 parts water. In other words, they are 1/10 the concentration of the original mineral complex. And when these diluted solutions are used for their intended purpose—purifying, clarifying, and structuring drinking water—the amount required is extremely small: typically 1 teaspoon (5 mL) per gallon, which further reduces the concentration to roughly 1:1000th of pure Themarox.
At that level of dilution, “treated water,” much like PHSS, contains negligible mineral content from a nutritional standpoint. It may produce exceptionally clean, structured, high-conductivity water, but it is not a source of dietary minerals in any meaningful supplementation sense.
That said, this is precisely where a new frontier may be emerging.
Several researchers—including Hisatake Nojima (See Chapter 18A)—experimented historically with adding measured volumes of diluted Themarox-derived mineral solutions to increase mineral intake. Nojima documented his observations extensively in a series of books, describing the patterns he witnessed and the hypotheses he formulated over time. His work is unusually detailed and certainly worthy of further scientific study.
This is why I have begun collaborating with mineral experts and practitioners to explore the possibility of developing a future dietary supplement derived from Themarox. At this early stage, the formulation, dosage, and intended use have not yet been determined, and any potential applications would require rigorous research, independent evaluation, and clear regulatory pathways before anything could be offered to the public.
Conclusion: The Contrast Between Inorganic Minerals And Plant-Based Substitutes
Everything alive is built from the Earth’s crust. Carbon, iron, magnesium, sulfur — all of it comes from stone. Life doesn’t create matter; it organizes it. Every leaf, cell, and structure is just mineral matter arranged into living form.
That’s why “organic” versus “inorganic” is a false choice. Plants don’t make minerals; they borrow them. When you take plant- or seaweed-based minerals, you’re receiving a version that has already moved through a biological system— bound to carbon, reshaped, and altered by the plant’s chemistry.
Geologic minerals, by contrast — ionic, sulfated, and unbound — are the elemental originals. They carry the natural charges and properties that allow them to interact with water, influence its structure, and take part in the geological processes that shape springs, rivers, and oceans.
So, at the end of this review comparing ocean, lake, humic, salt, and plant mineral sources, remember the first principle:
Organic matter is simply mineral matter rearranged by life.
To understand life at its foundation, you beginwith the minerals themselves.
Next: Chapter 17: Aluminum—From Feared Toxin to Forgotten Ally
P.S. If you’re curious about the volcanic-mineral water purification and structuring product that we derived from Shimanishi’s Themarox, you can find it at Aurmina.com.
Upcoming Book Publications
Yup — not one, but two books are dropping from yours truly (at the same time? What?)
If, instead of (or in addition to) this Substack version, you prefer the feel of a real book—or the smell of paper—or like to give holiday gifts, pre-order From Volcanoes to Vitality, my grand mineral saga, shipping before Christmas.
And if you want to read (or gift) another chronicle of suppression, science, and survival, grab The War on Chlorine Dioxide—the sequel you didn’t see coming—shipping mid-January. On this one, I say: “Buy it before they ban it.” Hah!.
© 2025 Pierre Kory. All rights reserved.
This chapter is original material and protected under international copyright law. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the author.








This was a great chapter for me. In the business of bioremediation and much lesser discussed; mineral remediation- of poisoned soils and water sources, or simply the depleted farms capes dominating the footprints of modern agriculture . It's often difficult to offer affordable broad acre remedies to many clients, who overwhelmingly call in crisis mode , rather than with prevention in mind.
Deep soil and watershed repair can be costly if replacement of missing factors is evident. This step often requires careful observation , not necessarily lab generated values .
Plants and animals are always displaying messages of their well being or distress , we often fail to see it and act in a timely manner.
Diverse cover cropping, generously nourished with as many perceived missing minerals and beneficial elements, as if it IS the primary cash crop is not widely embraced. For many farmers, cover cropping is often seen as a cash flow loss. Yet once the soil's skin is intact with vegetation, the return on investment slow as it may seem, provides deep rewards.
It has been said : There is a pecking order in soil systems :
First feed the soil micro and macro biota a broad buffet.
Plants eat second ,
Livestock and eventually humanity eats last .. IF we have done a good job of setting the table with a light foot print to avoid compaction and collapse of soil structure.
Thus promoting deep soil respiration , often found to be the limiting factor on so many farms when fertilizers , "recreational tillage" and institutional / salesman style advice fails to achieve lasting results.
Apologies for the mouthful. Much bubbles up in this topic. Notes from field observations .
Mark F
I'm so glad you included this chapter. I was wondering about the other sources of minerals like humic/fulvic and where they fit in with Themarox.